Public Notification: Pro ArthMax Contains Several Hidden Drug Ingredients
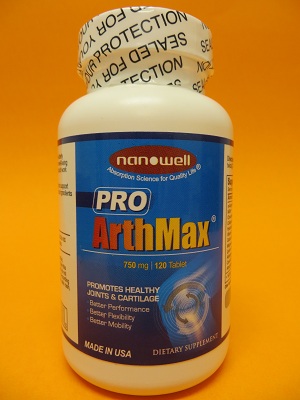
FDA laboratory analysis confirmed that Pro ArthMax contains the active ingredients diclofenac, ibuprofen, naproxen, indomethacin, nefopam, and chlorzoxazone.
- Diclofenac, ibuprofen, naproxen, and indomethacin are non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs may cause increased risk of cardiovascular events, such as heart attack and stroke, as well as serious gastrointestinal damage including bleeding, ulceration, and fatal perforation of the stomach and intestines.
- Chlorzoxazone is a muscle relaxant that is only available by prescription. Chlorzoxazone may cause drowsiness, dizziness, and lightheadedness, which may impair the ability to perform certain tasks, such as driving a motor vehicle or operating machinery.
- Nefopam is a non-narcotic pain relieving drug that is not approved for marketing in the U.S. Because nefopam is not FDA-approved, safety or efficacy has not been established. In literature, adverse events such as, rapid heart rate, sweating, dizziness, confusion, hallucinations, and seizures have been reported with nefopam use.
These hidden drug ingredients may interact with other medications and significantly increase the risk of adverse events, particularly when consumers already may be using NSAID-containing products.
Pro ArthMax is labeled in English, but also promoted to the Korean-speaking community as “프로알쓰맥스.”
Pro ArthMax is distributed by Human Science Foundation and is sold on various websites, including www.nanowellusa.com, and in some retail stores.
Consumers should stop using this product immediately and throw it away. Consumers should consult a health care professional as soon as possible if they have experienced any negative side effects, such as unusually dark stools or urine, stomach pain, increased bruising, other signs of bleeding, confusion, sedation, hallucinations, and seizures.
Health care professionals and patients are encouraged to report adverse events or side effects related to the use of this product to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.fda.gov/MedWatch/report.htm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
Note: This notification is to inform the public of a growing trend of dietary supplements or conventional foods with hidden drugs and chemicals. These products are typically promoted for sexual enhancement, weight loss, and body building, and are often represented as being “all natural.” FDA is unable to test and identify all products marketed as dietary supplements on the market that have potentially harmful hidden ingredients. Consumers should exercise caution before purchasing any product in the above categories.
Please refer to the links below for more information:
Subscribe to the RSS feed: http://www.fda.gov/AboutFDA/ContactFDA/StayInformed/RSSFeeds/TDS/rss.xml

