Public Notification: PAYA Dietary Supplement Product contains hidden drug ingredient
[6-7-2018] The Food and Drug Administration (FDA) is advising consumers not to purchase or use PAYA Dietary Supplement Product, a product promoted for weight loss. This product was identified by FDA during an examination of international mail shipments.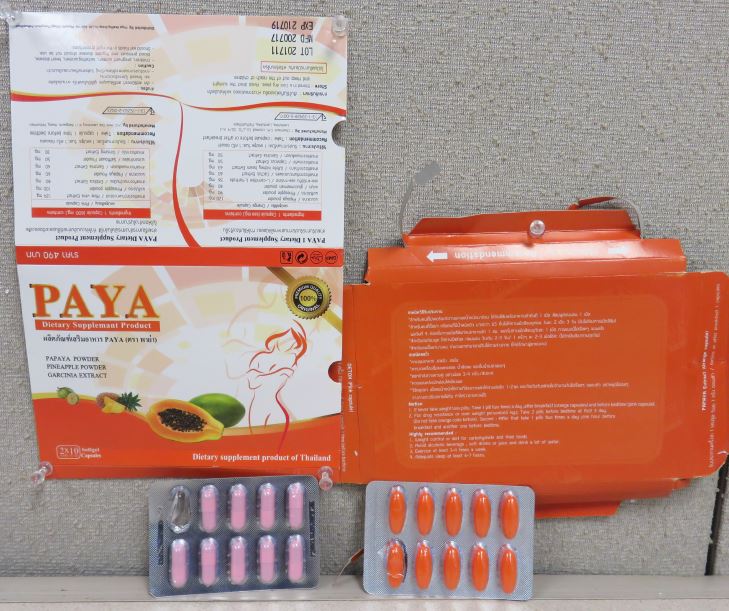
FDA laboratory analysis confirmed that PAYA Dietary Supplement Product contains sibutramine. Sibutramine is a controlled substance that was removed from the market in October 2010 for safety reasons.
This product poses a threat to consumers because sibutramine is known to substantially increase blood pressure and/or heart rate in some patients and may present a significant risk for patients with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke. This product may also interact, in life-threatening ways, with other medications a consumer may be taking.
Health care professionals and patients should report adverse events or side effects related to the use of this product to FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report online MedWatch Online Voluntary Reporting Form, or:
- Download and complete the form, then submit it via fax at 1-800-FDA-0178.
Note: This notification is to inform the public of a growing trend of dietary supplements or conventional foods with hidden drugs and chemicals. These products are typically promoted for sexual enhancement, weight loss, and body building and are often represented as being “all natural.” FDA is unable to test and identify all products marketed as dietary supplements that have potentially harmful hidden ingredients. Consumers should exercise caution before purchasing any product in the above categories.
For more information:

