Section 804 Importation Program (SIP) Proposals and FDA’s Review Process
Section 804 of the Federal Food, Drug and Cosmetic Act allows states and Indian tribes to import certain prescription drugs from Canada to significantly reduce the cost of these drugs without imposing additional risk to public health and safety.
FDA regulations at 21 C.F.R. part 251 describe the requirements necessary for a sponsor of a SIP to demonstrate that their importation program will result in a significant reduction in the cost of eligible prescription drugs to the American consumer without posing any additional risk to the public’s health and safety.
FDA has developed a resource, Tips for SIPs, which provides information to assist sponsors as they work to develop and implement a SIP proposal. A small entity compliance guide is available to help with developing a proposal.
Visit responses to comments in the rulemaking for more information.
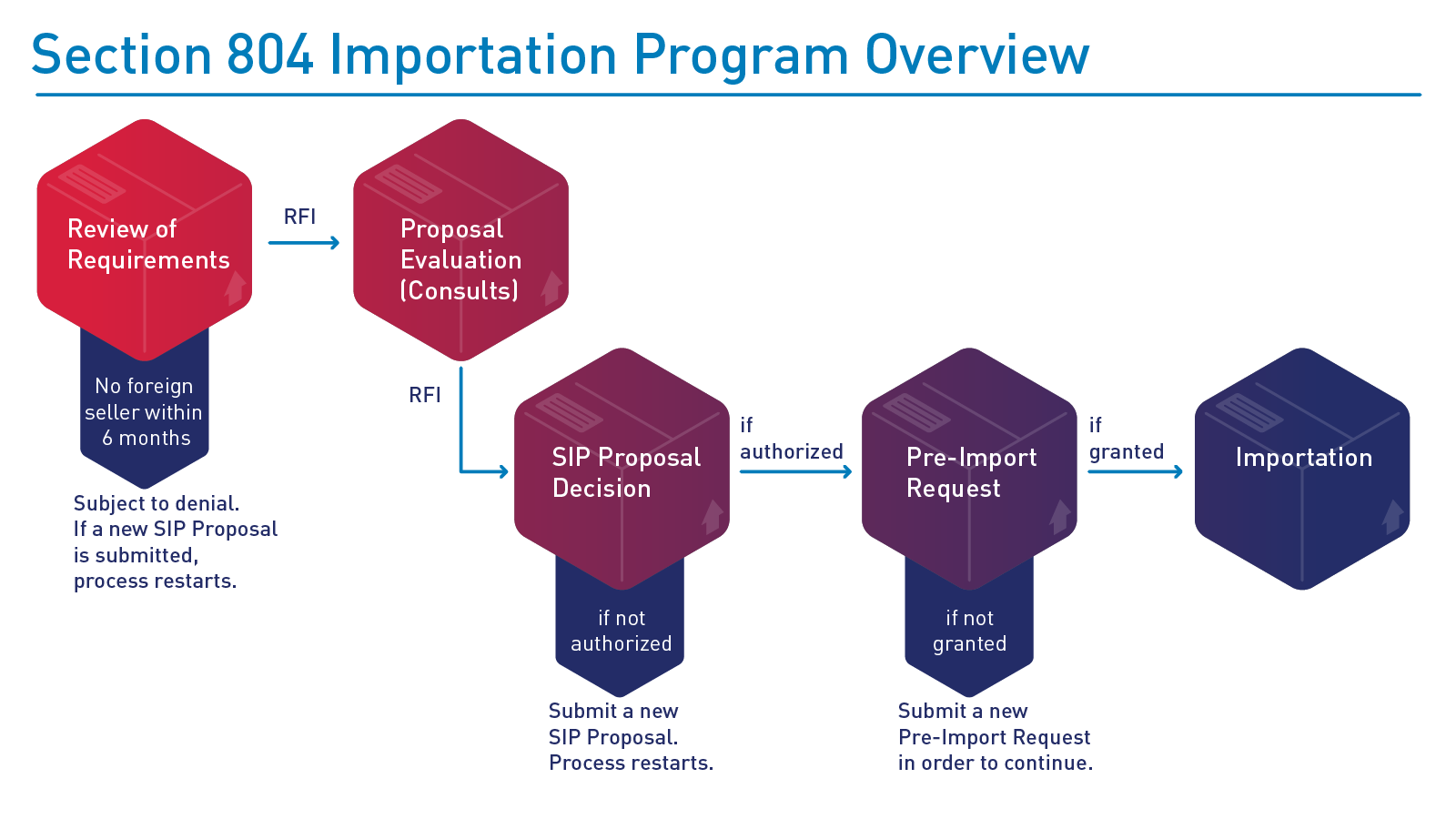
FDA SIP review process
FDA’s evaluation of SIP proposals may include requests for additional information necessary to ensure the proposal meets the requirements in the statute and final rule. The agency offers states and tribes the opportunity to submit a draft proposal for pre-review and meet with the agency to obtain initial feedback from FDA prior to formally submitting a proposal.
The following is a depiction of FDA’s SIP proposal evaluation and potential implementation process: