Drug Trials Snapshots: COPIKTRA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the COPIKTRA Package Insert for complete information.
COPIKTRA (duvelisib)
co-PIK-trah
Verastem, Inc
Approval date: September 24, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
COPIKTRA is a drug used to treat adult patients with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and follicular lymphoma (FL) who have received at least two prior treatments that did not work or are no longer working.
CLL, SLL, and FL are forms of blood cancer.
How is this drug used?
COPIKTRA is taken as one capsule by mouth twice daily for 28-day treatment cycles.
What are the benefits of this drug?
In adult patients with CLL or SLL, COPIKTRA may delay disease worsening. Patients treated with COPIKTRA lived about 16 months without disease worsening (progression) in comparison to patients treated with comparator drug (ofatumumab) and lived about 9 months without disease worsening.
Among 83 patients with FL who were treated with COPIKTRA, 35 patients (42%) had either complete or partial tumor shrinkage (response).
COPIKTRA for treatment of adult patients with FL was approved under FDA’s accelerated approval program, which provides earlier patient access to a new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
The figures below summarize efficacy results for the evaluated patients in Trials 1and 2. The primary outcome was progression free survival (PFS) and overall response rate (ORR) in Trial 1 and ORR in Trial 2.
Table 2. Efficacy in CLL or SLL After At least Two Prior Therapies (Trial 1)
| Outcome per IRC | COPIKTRA N = 95 |
Ofatumumab N = 101 |
|
|---|---|---|---|
| PFS | |||
| Number of events, n (%) | 55 (58) | 70 (69) | |
| Progressive disease | 44 | 62 | |
| Death | 11 | 8 | |
| Median PFS (SE), months a | 16.4 (2.1) | 9.1 (0.5) | |
| Hazard Ratio (SE), b COPIKTRA/ofatumumab |
0.40 (0.2) | ||
| Response Rate | |||
| ORR, n (%)c | 74 (78) | 39 (39) | |
|
CR |
0 (0) | 0 (0) | |
| PR | 74 (78) | 39 (39) | |
| Difference in ORR, % (SE) | 39 (6.4) | ||
Abbreviations: CI = confidence interval; CR = complete response; IRC = Independent Review Committee; PFS = progression-free survival; PR = partial response; SE = standard error
a Kaplan-Meier estimate
b Standard Error of ln(hazard ratio) = 0.2
c IWCLL or revised IWG response criteria, with modification for treatment-related lymphocytosis
COPIKTRA Prescribing Information
Table 3. Efficacy in Patients with Relapsed or Refractory FL (Trial 2)
| Endpoint | FL N = 83 |
|---|---|
| ORR, n (%) a | 35 (42) |
| 95% CI | (31, 54) |
| CR, n (%) | 1 (1) |
| PR, n (%) | 34 (41) |
| Duration of response | |
| Range, months | 0.0+ to 41.9+ |
| Patients maintaining response at 6 months, n/N (%) | 15/35 (43) |
| Patients maintaining response at 12 months, n/N (%) | 6/35 (17) |
Abbreviations: CI = confidence interval; CR = complete response; IRC = Independent Review Committee; ORR = overall response rate; PR = partial response
a Per IRC according to Revised International Working Group criteria
+ Denotes censored observation
COPIKTRA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: COPIKTRA worked similarly in men and women.
- Race: The majority of patients were White. Therefore, differences in how well the drug works among races could not be determined.
- Age: COPIKTRA worked similarly among patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by sex, race and age. Racial subgroup differences were investigated between White race and all other races combined since the majority of patients was White.
Table 4. Demographic Subgroup Analysis of Progression Free Survival (Trial 1)
| Event/Total | Median | Hazard Ratio (SE)1 | ||||
|---|---|---|---|---|---|---|
| Subgroup | Total | COPIKTRA | ofatumumab | COPIKTRA | ofatumumab | SE |
| Sex | ||||||
| Men | 115 | 38/59 | 35/56 | 14.7 | 9.0 | 0.47 (0.25) |
| Women | 81 | 17/36 | 35/45 | 27.6 | 9.2 | 0.29 (0.32) |
| Race | ||||||
| White | 183 | 53/90 | 66/93 | 16.4 | 9.1 | 0.39 (0.20) |
| All Other Races | 7 | 2/2 | 3/5 | 9.3 | 11.2 | 2.00 (1.03) |
| Age Group | ||||||
| <65 years | 137 | 38/68 | 47/69 | 16.4 | 9.2 | 0.38 (0.24) |
| ≥65 years | 137 | 38/68 | 47/69 | 16.4 | 9.2 | 0.38 (0.24) |
1 Unstratified Cox proportional hazards model.
2 All Other Races includes non-White and not reported
FDA Review
Table 5. Demographic Subgroup Analysis of Overall Response Rate per IRC (Trial 2)
| Follicular Lymphoma | Overall | |||||
|---|---|---|---|---|---|---|
| Subgroup | Total | ORR, n (%) | SE | Total | ORR, n (%) | SE |
| Sex | ||||||
| Men | 56 | 25 (45) | 0.06 | 88 | 43 (49) | 0.06 |
| Women | 27 | 10 (37) | 0.10 | 116 | 54 (47) | 0.08 |
| Race | ||||||
| White | 74 | 29 (39) | 0.06 | 116 | 54 (47) | 0.04 |
| All Other Races | 7 | 4 (57) | 0.18 | 11 | 5 (46) | 0.16 |
| Age Group | ||||||
| <65 years | 43 | 21 (49) | 0.08 | 64 | 33 (52) | 0.06 |
| ≥65 years | 40 | 14 (35) | 0.08 | 65 | 28 (43) | 0.06 |
FDA Review
What are the possible side effects?
COPIKTRA may cause serious side effects that can lead to death including infections, diarrhea or inflammation of the intestines, skin reactions, and inflammation of the lungs. Other serious side effects include abnormal liver blood tests and low white blood cell counts (neutropenia).
The most common side effects of COPIKTRA are diarrhea, inflammation of the intestines, low white blood cell count (neutropenia), rash, tiredness, fever, cough, nausea, upper respiratory infection, pneumonia, bone and muscle pain, and low red blood cell count (anemia).
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in adult patients with CLL, SLL, or FL in combined trials (safety population).
Table 6. Common Adverse Reactions (≥ 10% Incidence) in Patients with B-cell Malignancies Receiving COPIKTRA
| Adverse Reactions | COPIKTRA 25 mg BID (N = 442) |
|
|---|---|---|
| Any Grade n (%) |
Grade ≥ 3 n (%) |
|
| Blood and lymphatic system disorders | ||
| Neutropenia † | 151 (34) | 132 (30) |
| Anemia † | 90 (20) | 48 (11) |
| Thrombocytopenia † | 74 (17) | 46 (10) |
| Gastrointestinal disorders | ||
| Diarrhea or colitis †a | 222 (50) | 101 (23) |
| Nausea M† | 104 (24) | 4 (< 1) |
| Abdominal pain | 78 (18) | 9 (2) |
| Vomiting | 69 (16) | 6 (1) |
| Mucositis | 61 (14) | 6 (1) |
| Constipation | 57 (13) | 1 (< 1) |
| General disorders and administration site conditions | ||
| Fatigue † | 126 (29) | 22 (5) |
| Pyrexia | 115 (26) | 7 (2) |
| Hepatobiliary disorders | ||
| Transaminase elevation †b | 67 (15) | 34 (8) |
| Infections and infestations | ||
| Upper respiratory tract infection † | 94 (21) | 2 (< 1) |
| Pneumonia †c | 91 (21) | 67 (15) |
| Lower respiratory tract infection † | 46 (10) | 11 (3) |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 63 (14) | 2 (< 1) |
| Edema † | 60 (14) | 6 (1) |
| Hypokalemia † | 45 (10) | 17 (4) |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal pain † | 90 (20) | 6 (1) |
| Arthralgia | 46 (10) | 1 (< 1) |
| Nervous system disorders | ||
| Headache † | 55 (12) | 1 (< 1) |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough † | 111 (25) | 2 (< 1) |
| Dyspnea † | 52 (12) | 8 (2) |
| Skin and subcutaneous tissue disorders | ||
| Rash †d | 136 (31) | 41 (9) |
† Grouped term for reactions with multiple preferred terms
a Diarrhea or colitis includes the preferred terms: colitis, enterocolitis, colitis microscopic, colitis ulcerative, diarrhea, diarrhea hemorrhagic
b Transaminase elevation includes the preferred terms: alanine aminotransferase increased, aspartate aminotransferase increased, transaminases increased, hypertransaminasemia, hepatocellular injury, hepatotoxicity
c Pneumonia includes the preferred terms: All preferred terms containing "pneumonia" except for "pneumonia aspiration"; bronchopneumonia, bronchopulmonary aspergillosis
d Rash includes the preferred terms: dermatitis (including allergic, exfoliative, perivascular), erythema (including multiforme), rash (including exfoliative, erythematous, follicular, generalized, macular & papular, pruritic, pustular), toxic epidermal necrolysis and toxic skin eruption, drug reaction with eosinophilia and systemic symptoms, drug eruption, Stevens-Johnson syndrome
COPIKTRA PRESCRIBING INFORMATION
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients were White. Therefore, differences the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar among patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most common adverse reaction, diarrhea/colitis, by sex and age.
Table 7. Subgroup Analysis of Diarrhea/Colitis (safety population)
| Demographic Characteristic | COPIKTRA n/N (%) |
|---|---|
| Sex | |
| Men | 129/289 (45) |
| Women | 93/153 (61) |
| Age Group | |
| < 65 years | 81/172 (47) |
| > 65 years | 141/270 (52) |
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved COPIKTRA based on evidence from four clinical trials (Trial 1/NCT02004522, Trial 2/NCT02204982, Trial 3/NCT01476657 Trial 4/NCT02049515) of 442 adult patients with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), or follicular lymphoma (FL) who received at least two prior treatments that did not work or was no longer working.
Trials were conducted at 92 sites in Australia, Canada, Europe, New Zealand, and the United States.
The population that provided data for side effects of COPIKTRA (safety population) is presented below. Demographics of the patients who provided data for evaluation of benefits (efficacy population) are presented in Table 9, under the MORE INFO section.
Figure 1 summarizes how many men and women were in the clinical trials used to evaluate safety.
Figure 1. Baseline Demographics by Sex
FDA Review
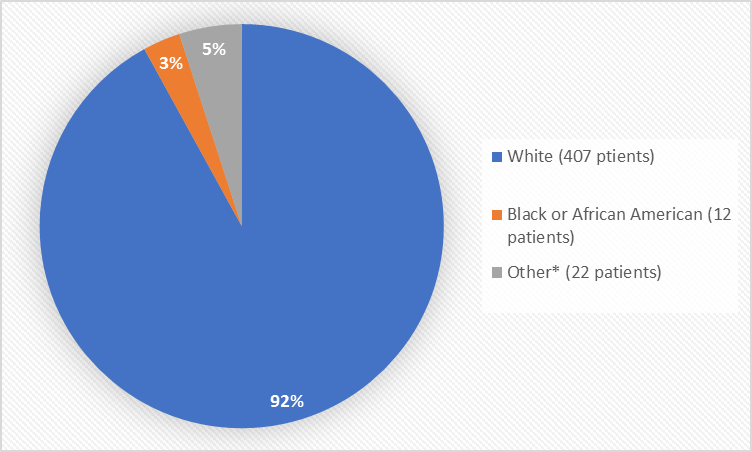
Figure 2 summarizes the percentage of patients by race in the clinical trials used to evaluate safety.
Figure 2. Baseline Demographics by Race
*Other includes Asian, American Indian or Alaska Native, Not Reported
FDA Review
Table 1. Demographics of Trials by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 407 | 92% |
| Black or African American | 12 | 3% |
| Asian | 1 | Less than 1% |
| American Indian or Alaska Native | 1 | Less than 1% |
| Other* | 15 | 3% |
| Not Reported | 6 | 1% |
Clinical Trial Data
Figure 3 summarizes the percentage of patients by age in the clinical trials used to evaluate safety.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The tables below summarizes demographics of the safety and efficacy populations.
Table 8. Baseline Demographics of the Safety Population/strong>
| Demographic Characteristic | COPIKTRA N=442 |
|---|---|
| Sex, n (%) | |
| Men | 289 (65%) |
| Women | 153 (35%) |
| Race, n (%) | |
| White | 407 (92%) |
| Black or African American | 12 (3) |
| Asian | 1 (< 1) |
| American Indian or Alaska Native | 1 (< 1) |
| Other | 15 (3) |
| Not Reported | 6 (1) |
| Age (years) | |
| Median | 67 |
| Min, Max | 30, 90 |
| Age Group, n (%) | |
| < 65 years | 172 (39) |
| > 65 years | 270 (61) |
| Ethnicity, n (%) | |
| Hispanic | 56 (13) |
| Non-Hispanic | 386 (87) |
| Region, n (%) | |
| Europe | 262 (59) |
| Canada | 9 (2) |
| United States | 148 (33) |
| Other | 23 (5) |
Clinical Trial Data
Table 9. Baseline Demographics of the Efficacy Population
| Demographic Characteristic | Trial 1 | Trial 2 | |
|---|---|---|---|
| COPIKTRA N=95 |
Ofatumumab N=101 |
COPIKTRA N=83 |
|
| Sex, n (%) | |||
| Men | 59 (62%) | 56 (55) | 56 (68) |
| Women | 36 (38%) | 45 (45) | 27 (32) |
| Race, n (%) | |||
| White | 90 (95%) | 93 (92) | 74 (89) |
| Black or African American | 0 (0) | 1 (1) | 3 (4) |
| Asian | 0 (0) | 0 (0) | 1 (1) |
| American Indian or Alaska Native | 0 (0) | 0 (0) | 1 (1) |
| Other | 2 (2) | 4 (4) | 4 (5) |
| Not Reported | 3 (3) | 3 (3) | 0 (0) |
| Age (years) | |||
| Median | 70 | 68 | 64 |
| Min, Max | 40, 90 | 44, 89 | 30, 82 |
| Age Group, n (%) | |||
| < 65 years | 27 (28) | 32 (32) | 43 (52) |
| > 65 years | 68 (72) | 69 (68) | 40 (48) |
| Ethnicity, n (%) | |||
| Hispanic | 2 (2) | 2 (2) | 9 (11) |
| Non-Hispanic | 93 (98) | 99 (98) | 74 (89) |
| Region, n (%) | |||
| Europe | 71 (75) | 82 (81) | 45 (54) |
| United States | 18 (19) | 9 (9) | 25 (30) |
| Other | 6 (6) | 10 (10) | 13 (16) |
Clinical Trial Data
How were the trials designed?
The benefit and side effects of COPIKTRA were evaluated in four clinical trials of adult patients 30 – 90 years of age with CLL, SLL, or FL.
Trial 1 enrolled adult patients with chronic lymphocytic leukemia and small lymphocytic lymphoma after at least one prior treatment that did not work or was no longer working. Patients were assigned to receive COPIKTRA twice daily by mouth or ofatumumab for 7 weekly intravenous infusions (injected into the vein) followed by 4 monthly intravenous infusions. Treatment continued until either disease worsened or patients experienced unacceptable toxicity. The benefit of COPIKTRA was assessed based on the length of time that the disease did not get worse, comparing patients in the COPIKTRA and ofatumumab groups.
Trial 2 enrolled adult patients with follicular lymphoma that did not respond to or no longer responded to treatment with rituximab (a drug used to treat CLL or FL), chemotherapy (chemicals used to treat cancer) or radio-immunotherapy (treatment that uses the body’s own immune system to combat cancer). Patients received COPIKTRA twice daily by mouth during a 28-day cycle. Treatment continued until either disease worsened or patients experienced unacceptable toxicity. The benefit of COPIKTRA was evaluated by measuring how many patients had complete or partial tumor shrinkage (response) and how long that response lasted.
Trial 3 enrolled adult patients with advanced blood cancers. Patients received increasing doses of COPIKTRA based on toxicity, over 28-day treatment cycles. Patients in Trial 3 were primarily evaluated for side effects.
Trial 4 enrolled patients from Trial 1 who experienced worsening of disease. Patients who previously received ofatumumab in Trial 1 were assigned to receive COPIKTRA twice daily by mouth until the disease worsened, they discontinued participation in the trial or started another treatment. Patients who previously received COPIKTRA in Trial 1, were assigned to receive ofatumumab as 7 weekly intravenous infusions followed by four monthly intravenous infusions. Patients in Trial 4 were primarily evaluated for side effects.
How were the trials designed?
The safety and efficacy of COPIKTRA were established in 4 clinical trials.
Trial 1 was an open-label, randomized, superiority trial designed to compare COPIKTRA to ofatumumab in adult patients with chronic lymphocytic leukemia or small lymphocytic lymphoma whose disease is relapsed or refractory after at least one prior therapy. Patients were randomized to receive COPIKTRA 25 mg twice daily until disease progression or unacceptable toxicity or ofatumumab for seven 28-day cycles. Ofatumumab was administered initially at a starting dose of 300 mg ofatumumab on Day 1 followed by seven weekly doses of 2000 mg. Thereafter, patients received 2000 mg ofatumumab once every month for four months. The primary efficacy endpoint was the progression-free survival (PFS) as assessed by an Independent Review Committee (IRC).
Trial 2 was an open-label, single arm trial in adult patients with follicular lymphoma whose disease is refractory to rituximab and to either chemotherapy or radio-immunotherapy. Patients received COPIKRA 25 mg twice daily over the course of 28-day treatment cycles until disease progression or unacceptable toxicity. The primary efficacy endpoint was the overall response rate defined as the best response of complete or partial response according to the 2007 revised International Working Group (IWG) Criteria.
Trial 3 was a dose-escalation trial in adult patients with advanced hematologic malignancies. COPIKTRA was supplied as 1 mg, 5 mg, 25 mg, and 100 mg formulated capsules. Patients were primarily evaluated for adverse events.
Trial 4 was an open-label, two-arm, extension trial. Patients who previously received ofatumumab in Trial 1, received COPIKTRA 25 mg twice daily in a 21- day cycle for Cycle 1 followed by 28- day treatment cycles thereafter until disease progression, discontinuation from study participation, or start of subsequent therapy. Patients who previously received COPIKTRA in Trial 1, received ofatumumab at a starting dose of 300 mg on Day 1 followed by seven weekly doses of 2000 mg. Thereafter, patients received 2000 mg ofatumumab once every month for four months unless disease progression or unacceptable toxicity.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION