Drug Trials Snapshots: BIMZELX
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the BIMZELX Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
BIMZELX (bimekizumab-bkzx)
bim zel’ex
UCB, Inc.
Original Approval date: October 17, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BIMZELX is an anti-interleukin (IL)-17A and IL-17F monoclonal antibody that is indicated for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
How is this drug used?
BIMZELX is a solution for subcutaneous injection that is taken as 320 mg (given as two subcutaneous injections of 160 mg each) at Weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥120 kg, a dosage of 320 mg every 4 weeks after Week 16 may be considered.
Who participated in the clinical trials?
The FDA approved BIMZELX based on evidence of safety and efficacy from two placebo-controlled clinical trials which included a total of 839 patients with moderate to severe plaque psoriasis. The trials were conducted at 182 sites in 13 countries including Australia, Belgium, Canada, Germany, Hungary, Italy, Japan, Poland, Republic of Korea, Russian Federation, Taiwan, the United Kingdom, and the United States.
In addition, safety analyses were performed on the combined results of these two placebo-controlled clinical trials. Supportive safety analyses were performed using data from two additional active-controlled clinical trials in a total of 1,169 patients.
How were the trials designed?
The benefit and side effects of BIMZELX were evaluated in two clinical trials of 839 patients ages 18 years and older with moderate to severe plaque psoriasis.
Both trials evaluated a dose of 320 mg of BIMZELX. Trial Ps-1 was a 52-week, multicenter, randomized, double-blind, placebo- and active comparator-controlled, parallel-group, phase 3 trial with ustekinumab as a comparator. In this trial, at Week 16 both subjects previously receiving BIMZELX 320 mg every four weeks and subjects previously receiving placebo were treated with BIMZELX 320 mg every four weeks. Subjects who were treated with ustekinumab continued to receive ustekinumab after Week 16.
Trial Ps-2 was a 56-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 trial. At Week 16, subjects previously receiving BIMZELX 320 mg every four weeks who had achieved a 90% improvement on their Psoriasis Area and Severity Index (PASI) score were rerandomized to treatment with BIMZELX 320 mg every four weeks, BIMZELX 320 mg every eight weeks, or placebo (i.e., treatment with BIMZELX was withdrawn). Subjects previously treated with placebo continued placebo at Week 16. Subjects in the placebo group who fell below 75% improvement in their PASI response after Week 16 were retreated with BIMZELX 320 mg every four weeks.
The benefit of BIMZELX compared to placebo was assessed after 16 weeks of treatment using the Investigator’s Global Assessment (IGA) scale that measures the severity of disease on a scale of 0 to 4 and the PASI for both trials.
How were the trials designed?
The safety and efficacy of BIMZELX was evaluated in two randomized, double-blind, placebo-controlled trials. One of the trials also included an active comparator (ustekinumab). The trials included subjects ages 18 years of age and older with moderate to severe plaque psoriasis, defined as a body surface area involvement of ≥10%, an IGA score of ≥3 (“moderate”) in the overall assessment of psoriasis on a severity scale of 0 to 4, and a PASI score ≥12.
Both trials assessed the following coprimary endpoints at Week 16:
- Proportion of subjects achieving IGA score of 0 (“clear”) or 1 (“almost clear”) with at least a 2-grade improvement from Baseline to Week 16
- Proportion of subjects achieving ≥90% improvement in PASI score from Baseline to Week 16
BIMZELX was statistically superior to placebo (p-values <0.001) for each coprimary efficacy endpoint in both trials.
DEMOGRAPHICS SNAPSHOT
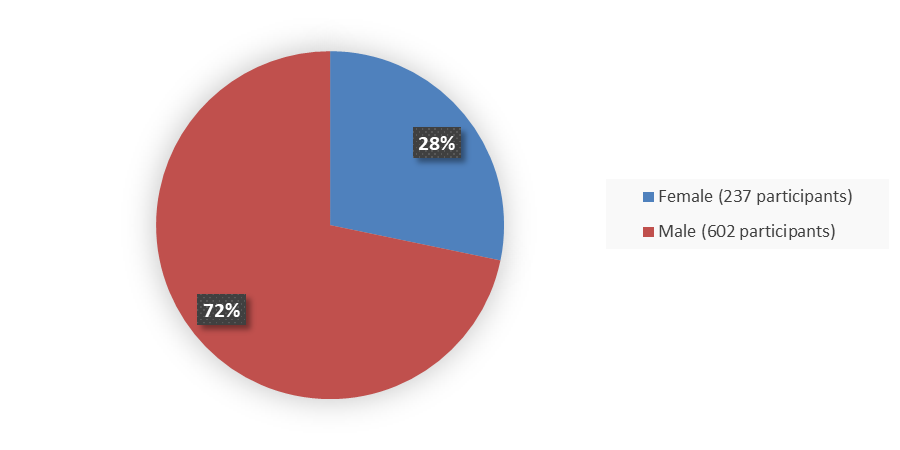
Figure 1 summarizes how many males and females were enrolled in the combined clinical trials (Pool S1) used to evaluate the safety and efficacy of BIMZELX.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
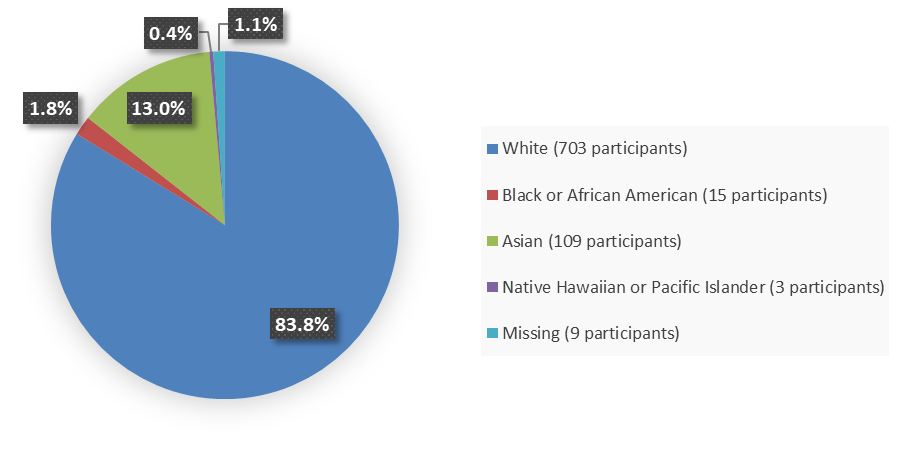
Figure 2 summarizes the percentage of patients by race enrolled in the combined clinical trials used to evaluate the safety and efficacy of BIMZELX.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
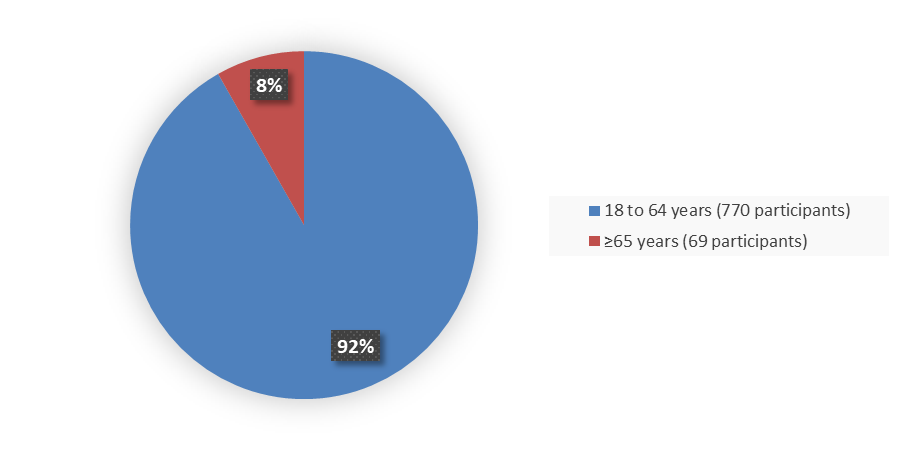
Figure 3 summarizes the percentage of patients by age group were in the combined trials used to evaluate the safety and efficacy of BIMZELX.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
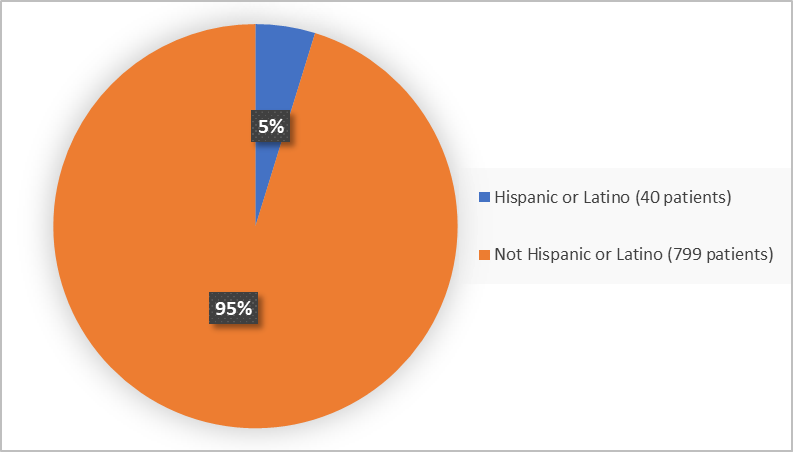
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trial used to evaluate the side effects of BIMZELX.
Figure 4. Baseline Demographics for Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 summarizes demographics for patients enrolled in Trials Ps-1 and Ps-1.
Table 1. Demographics for Trials Ps-1 and Ps-2 (RS*)
Demographic | Trial Ps-1 | Trial Ps-2 | |||
|---|---|---|---|---|---|
BIMZELX | Uste | Placebo | BIMZELX | Placebo | |
| Age, years | |||||
| Age group, years, n (%) | |||||
| Sex, n (%) | |||||
| Race, n (%) | |||||
| Mean (SD) | 45.2 (14.0) | 46.0 (13.6) | 49.7 (13.6) | 44.5 (12.9) | 43.5 (13.1) |
| Median | 43.0 | 47.0 | 50.0 | 45.0 | 42.0 |
| Range (min, max) | 18, 81 | 18, 79 | 19, 78 | 18, 81 | 18, 77 |
| <65 | 287 (89) | 145 (89) | 73 (88) | 328 (94) | 82 (95) |
| ≥65 | 34 (11) | 18 (11) | 10 (12) | 21 (6) | 4 (5) |
| Male | 229 (71) | 117 (72) | 60 (72) | 255 (73) | 58 (67) |
| Female | 92 (29) | 46 (28) | 23 (28) | 94 (27) | 28 (33) |
| White | 237 (74) | 120 (74) | 63 (76) | 324 (93) | 79 (92) |
| Black or African American | 9 (3) | 3 (2) | 0 (0) | 6 (2) | 0 (0) |
| Native Hawaiian or Pacific Islander | 1 (<1) | 1 (1) | 0 (0) | 2 (1) | 0 (0) |
| Asian | 71 (22) | 36 (22) | 20 (24) | 13 (4) | 5 (6) |
| Missing | 3 (1) | 3 (2) | 0 (0) | 4 (1) | 2 (2) |
Source: Adapted from FDA Review
* RS: all randomized subjects
Abbreviations: RS, randomized set; SD, standard deviation; Uste, ustekinumab
What are the benefits of this drug?
More patients achieved clear or almost clear skin after treatment with BIMZELX in comparison to those who were treated with placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 summarizes the efficacy results for the evaluated patients in two randomized, double-blind, placebo-controlled trials (Trial Ps-1 and Trial Ps-2).
Table 2. Efficacy Results at Week 16 in BIMZELX- or Placebo-Treated Adults With Plaque Psoriasis in Trial-Ps-1 and Trial-Ps-2
Parameter | Trial-Ps-1 | Trial-Ps-2 | ||
|---|---|---|---|---|
BIMZELX | Placebo | BIMZELX | Placebo | |
| IGA 0 or 1 (“clear” or “almost clear”)a | 270 (84) | 4 (5) | 323 (93) | 1 (1) |
| Difference % (95% CI) | 79 (73, 85) | 91 (88, 95) | ||
| PASI 90a | 273 (85) | 4 (5) | 317 (91) | 1 (1) |
| Difference % (95% CI) | 80 (74, 86) | 90 (86, 93) | ||
| IGA 0 (“clear”) | 188 (59) | 0 (0) | 243 (70) | 1 (1) |
| Difference % (95% CI) | 59 (53, 64) | 69 (64, 74) | ||
| PASI 100 | 188 (59) | 0 (0) | 238 (68) | 1 (1) |
| Difference % (95% CI) | 59 (53, 64) | 67 (62, 73) | ||
Source: BIMZELX Prescribing Information
a Co-primary endpoints
PASI 90: Responders who experienced ≥90% improvement in PASI from baseline
PASI 100: Responders who experienced 100% improvement in PASI from baseline
Abbreviations: CI, confidence interval; IGA, Investigator’s Global Assessment; PASI, Psoriasis Area and severity Index
In addition, a greater proportion of subjects randomized to BIMZELX achieved 75% improvement from baseline (PASI 75) at Week 4 in both trials compared to placebo. In Trial-Ps-1, 77% of subjects treated with BIMZELX achieved PASI 75 compared to 2% treated with placebo. In Trial-Ps-2, 76% of subjects treated with BIMZELX achieved PASI 75 compared to 1% treated with placebo.
Among subjects with Scalp IGA score of at least 2 at baseline, a greater proportion of subjects randomized to BIMZELX achieved Scalp IGA response (defined as a Scalp IGA score of 0 or 1 with a 2-grade improvement from baseline) at Week 16 in both trials compared to placebo. In Trial-Ps-1, 84% of subjects treated with BIMZELX achieved Scalp IGA response compared to 15% of placebo-treated subjects. In Trial-Ps-2, 92% of subjects treated with BIMZELX achieved Scalp IGA response compared to 7% of placebo-treated subjects.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: BIMZELX worked similarly in males or females.
- Race: BIMZELX worked similarly in White and patients of other races. However, the sample size for races other than White was relatively small.
- Age: The number of patients older than 65 years of age was limited. Therefore, differences in response between patients older and younger than 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Examination of age, sex, and race did not identify differences in response to BIMZELX among these subgroups at Week 16. Table 3 through Table 6 summarize efficacy results at Week 16 by age, race, and sex.
Table 3. IGA 0/1 at Week 16 by Age, Sex, and Race - Trial PS-1 (RS; NRI1)
Subgroups | BIMZELX | Placebo |
|
|---|---|---|---|
| Age, years | |||
| Sex | |||
| Race | |||
| 18 to 64 (287, 73) | 86 | 5 | 80 (74, 87) |
| ≥65 (34, 10) | 71 | 0 | 71 (55, 86) |
| Male (229, 60) | 85 | 5 | 80 (73, 87) |
| Female (92, 23) | 81 | 4 | 77 (66, 89) |
| White (237, 63) | 84 |
| 78 (70, 86) |
| Other (84, 20) | 83 | 0 | 83 (75, 91) |
| Overall | 84 | 5 | 79 (73, 85) |
Source: Adapted from FDA Review
1 RS: all randomized subjects; missing data are imputed using NRI
Abbreviations: CI, confidence interval; IGA, Investigator’s Global Assessment; n[BKZ], number of patients matching criteria in BIMZELX arm; n[P], number of patients matching criteria in placebo arm; NRI, non-responder imputation; RS, randomized set
Table 4. PASI-90 Response at Week 16 by Age, Sex, and Race - Trial PS-1 (RS; NRI1)
| Subgroups (n[BKZ], n[P]) | BIMZELX | Placebo |
|
|---|---|---|---|
| Age, years | |||
| Sex | |||
| Race | |||
| 18 to 64 (287, 73) | 86 | 5 | 81 (74, 87) |
| ≥65 (34, 10) | 73 | 0 | 73 (59, 88) |
| Male (229, 60) | 85 | 7 | 78 (71, 86) |
| Female (92, 23) | 85 | 0 | 85 (77, 92) |
| White (237, 63) | 85 | 5 | 80 (73, 87) |
| Other (84, 20) | 86 | 5 | 81 (69, 93) |
| Overall | 85 | 5 | 80 (74, 86) |
Source: Adapted from FDA Review
1 RS: all randomized subjects; missing data are imputed using NRI
Abbreviations: CI, confidence interval; n[BKZ], number of patients matching criteria in BIMZELX arm; n[P], number of patients matching criteria in placebo arm; NRI, non-responder imputation; PASI, Psoriasis Area and severity Index; RS, randomized set
Table 5. IGA 0/1 at Week 16 by Age, Sex, and Race – Trial PS-2 (RS; NRI1)
| Subgroups (n[BKZ], n[P]) | BIMZELX | Placebo |
|
|---|---|---|---|
| Age, years | |||
| Sex | |||
| Race | |||
| 18 to 64 (328, 82) | 93 | 1 | 91 (88, 95) |
| ≥65 (21, 4) | 90 | 0 | 90 (78, 100) |
| Male (255, 58) | 81 | 2 | 90 (86, 95) |
| Female (94, 28) | 94 | 0 | 94 (89, 99) |
| White (324, 79) | 93 | 1 | 92 (89,96) |
| Other (25, 7) | 80 | 0 | 80 (64, 96) |
| Overall | 93 | 1 | 91 (88, 95) |
Source: Adapted from FDA Review
1 RS: all randomized subjects; missing data are imputed using NRI
Abbreviations: CI, confidence interval; IGA, Investigator’s Global Assessment; n[BKZ], number of patients matching criteria in BIMZELX arm; n[P], number of patients matching criteria in placebo arm; NRI, non-responder imputation; RS, randomized set
Table 6. PASI-90 Response at Week 16 by Age, Sex, and Race – Trial PS-2 (RS; NRI1)
| Subgroups (n[BKZ], n[P]) | BIMZELX | Placebo |
|
|---|---|---|---|
| Age, years | |||
| Sex | |||
| Race | |||
| 18 to 64 (328, 82) | 91 | 1 | 90 (86, 94) |
| ≥65 (21, 4) | 86 | 0 | 86 (71, 100) |
| Male (255, 58) | 91 | 2 | 89 (85, 94) |
| Female (94, 28) | 91 | 0 | 91 (86, 97) |
| White (324, 79) | 92 | 1 | 90 (87, 94) |
| Other (25, 7) | 80 |
| 80 (64, 96) |
| Overall | 91 |
| 80 (86, 93) |
Source: Adapted from FDA Review
1 RS: all randomized subjects; missing data are imputed using NRI
Abbreviations: CI, confidence interval; n[BKZ], number of patients matching criteria in BIMZELX arm; n[P], number of patients matching criteria in placebo arm; NRI, non-responder imputation; PASI, Psoriasis Area and severity Index; RS, randomized set
What are the possible side effects?
- upper respiratory tract infections
- headache
- herpes simplex infections (cold sores in or around the mouth)
- small red bumps on your skin
- feeling tired
- fungal infections (oral thrush or infections in the mouth, throat, skin, nails, feet, or genitals)
- pain, redness, or swelling at injection site
- stomach flu (gastroenteritis)
- acne
Treatment with BIMZELX has been associated with suicidal thoughts and behavior, infections, liver laboratory abnormalities, and inflammatory bowel disease.
Patients should not receive live vaccines while being treated with BIMZELX.
What are the possible side effects (results of trials used to assess safety)?
Table 7 summarizes safety results up to Week 16 for the evaluated patients in the placebo-controlled clinical trials with BIMZELX.
Table 7. Adverse Reactions Occurring in ≥1% of Subjects With Plaque Psoriasis in the BIMZELX Group and More Frequently Than in the Placebo Group in Trials Ps-1 and Ps-2
Adverse Reaction | BIMZELX | Placebo |
|---|---|---|
| Upper respiratory infectiona | 102 (15) | 24 (14) |
| Oral candidiasisb | 61 (9) | 0 (0) |
| Headache | 22 (3) | 0 (0) |
| Injection site reactionsc | 19 (3) | 2 (1) |
| Tinea infectionsd | 18 (3) | 1 (1) |
| Gastroenteritise | 12 (2) | 0 (0) |
| Herpes simplex infectionsf | 9 (1) | 0 (0) |
| Acne | 8 (1) | 0 (0) |
| Folliculitis | 8 (1) | 0 (0) |
| Other Candida infectionsg | 7 (1) | 1 (1) |
| Fatigue | 7 (1) | 0 (0) |
Source: BIMZLEX Prescribing Information
a Upper respiratory infections include nasopharyngitis, upper respiratory tract infection, pharyngitis, rhinitis, viral upper respiratory tract infection, tonsillitis, sinusitis, pharyngitis streptococcal, pharyngitis bacterial, peritonsillar abscess, viral rhinitis, and influenza
b Oral candidiasis includes oral candidiasis, oropharyngeal candidiasis, oral fungal infection, fungal pharyngitis, and oropharyngitis fungal
c Injection site reactions include injection site reaction, injection site erythema, injection site pain, injection site edema, injection site bruising, and injection site swelling
d Tinea Infections include tinea pedis, fungal skin infection, tinea versicolor, tinea cruris, tinea infection, body tinea, and onychomycosis
e Gastroenteritis includes Enterovirus infection, gastroenteritis, gastroenteritis bacterial, and gastroenteritis viral
f Herpes simplex infections include herpes simplex and oral herpes
g Other Candida infections include vulvovaginal candidiasis, vulvovaginal mycotic infection, skin candida, and genital candidiasis.
Were there any differences in side effects of the clinical trials among sex, race, and age?
Although certain adverse reactions were more frequent in some subgroups, because of the small subgroup sample sizes, it cannot be concluded that these differences are clinically meaningful.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Adverse reactions through Week 16 of the placebo-controlled trials (Pool S1) by sex, age, and race are presented in Table 8, Table 9, and Table 10.
Table 8. Adverse Reactions Occurring in ≥1% of Subjects Treated With BIMZELX and More Frequently in BIMZELX Than Placebo, by Sex, Pool S1
Adverse Reaction | BIMZELX | Placebo | ||
|---|---|---|---|---|
Female | Male | Female | Male | |
| Upper respiratory infectionsa | 37 (19.9) | 65 (13.4) | 9 (17.6) | 15 (12.7) |
| Candida infectionsb | 23 (12.4) | 43 (8.9) | 1 (2.0) | 0 (0.0) |
| Headache | 8 (4.3) | 14 (2.9) | 0 (0.0) | 0 (0.0) |
| Injection site reactionsc | 8 (4.3) | 11 (2.3) | 1 (2.0) | 1 (0.8) |
| Tinea infectionsd | 1 (0.5) | 17 (3.5) | 0 (0.0) | 1 (0.8) |
| Gastroenteritise | 9 (4.8) | 20 (4.1) | 3 (5.9) | 3 (2.5) |
| Herpes simplex infectionsf | 5 (2.7) | 4 (0.8) | 0 (0.0) | 0 (0.0) |
| Acne | 4 (2.2) | 4 (0.8) | 0 (0.0) | 0 (0.0) |
| Folliculitis | 1 (0.5) | 7 (1.4) | 0 (0.0) | 0 (0.0) |
| Fatigue | 2 (1.1) | 5 (1.0) | 0 (0.0) | 0 (0.0) |
Source: Adapted from FDA Review
a Upper respiratory infections include nasopharyngitis, upper respiratory tract infection, pharyngitis, rhinitis, viral upper respiratory tract infection, tonsillitis, sinusitis, pharyngitis streptococcal, pharyngitis bacterial, peritonsillar abscess, viral rhinitis, and influenza
b Candida infections include oral candidiasis, oropharyngeal candidiasis, vulvovaginal candidiasis, vulvovaginal mycotic infection, oral fungal infection, oropharyngitis fungal, skin candida, genital candidiasis, and fungal pharyngitis
c Injection site reactions include injection site reaction, injection site erythema, injection site pain, injection site edema, injection site bruising, and injection site swelling
d Tinea infections include tinea pedis, fungal skin infection, tinea versicolor, tinea cruris, tinea infection, body tinea, and onychomycosis
e Gastroenteritis includes gastroenteritis, gastroenteritis viral, gastroenteritis bacterial, enterovirus infection, diarrhea, nausea, vomiting, enteritis, enterocolitis, and gastritis
f Herpes simplex infections include herpes simplex and oral herpes
Table 9. Adverse Reactions Occurring in ≥1% of Subjects Treated With BIMZELX and More Frequently in BIMZELX Than Placebo, by Age Group, Pool S1
Adverse Reaction | BIMZELX | Placebo | ||||
|---|---|---|---|---|---|---|
<40 Years | 40 to <65 Years | ≥65 Years | <40 Years | 40 to <65 Years | ≥65 Years | |
| Upper respiratory infectionsa | 50 (20.1) | 47 (12.8) | 5 (9.1) | 13 (24.1) | 10 (9.9) | 1 (7.1) |
| Candida infectionsb | 24 (9.6) | 32 (8.7) | 10 (18.2) | 0 (0.0) | 1 (1.0) | 0 (0.0) |
| Headache | 12 (4.8) | 9 (2.5) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection site reactionsc | 9 (3.6) | 8 (2.2) | 2 (3.6) | 2 (3.7) | 0 (0.0) | 0 (0.0) |
| Tinea infectionsd | 8 (3.2) | 9 (2.5) | 1 (1.8) | 0 (0.0) | 1 (1.0) | 0 (0.0) |
| Gastroenteritise | 14 (5.6) | 13 (3.6) | 2 (3.6) | 3 (5.6) | 2 (2.0) | 1 (7.1) |
| Herpes simplex infectionsf | 7 (2.8) | 1 (0.3) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Acne | 4 (1.6) | 4 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Folliculitis | 4 (1.6) | 4 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 4 (1.6) | 2 (0.5) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Source: Adapted from FDA Review
a Upper respiratory infections include nasopharyngitis, upper respiratory tract infection, pharyngitis, rhinitis, viral upper respiratory tract infection, tonsillitis, sinusitis, pharyngitis streptococcal, pharyngitis bacterial, peritonsillar abscess, viral rhinitis, and influenza
b Candida infections include oral candidiasis, oropharyngeal candidiasis, vulvovaginal candidiasis, vulvovaginal mycotic infection, oral fungal infection, oropharyngitis fungal, skin candida, genital candidiasis, and fungal pharyngitis
c Injection site reactions include injection site reaction, injection site erythema, injection site pain, injection site edema, injection site bruising, and injection site swelling
d Tinea infections include tinea pedis, fungal skin infection, tinea versicolor, tinea cruris, tinea infection, body tinea, and onychomycosis
e Gastroenteritis includes gastroenteritis, gastroenteritis viral, gastroenteritis bacterial, enterovirus infection, diarrhea, nausea, vomiting, enteritis, enterocolitis, and gastritis
f Herpes simplex infections include herpes simplex and oral herpes
Table 10. Adverse Reactions Occurring in ≥1% of Subjects Treated With BIMZELX and More Frequently in BIMZELX Than Placebo, by Race, Pool S1
Adverse Reaction | BIMZELX | Placebo | |||||
|---|---|---|---|---|---|---|---|
Asian | Black or | Other | White | Asian | Other | White | |
| Upper respiratory infectionsa | 14 (16.7) | 1 (6.7) | 3 (30.0) | 84 (15.0) | 2 (8.0) | 1 (50.0) | 21 (14.8) |
| Candida infectionsb | 7 (8.3) | 1 (6.7) | 0 (0.0) | 58 (10.3) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Headache | 1 (1.2) | 0 (0.0) | 0 (0.0) | 21 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection site reactionsc | 3 (3.6) | 0 (0.0) | 0 (0.0) | 16 (2.9) | 1 (4.0) | 0 (0.0) | 1 (0.7) |
| Tinea infectionsd | 5 (6.0) | 0 (0.0) | 0 (0.0) | 13 (2.3) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Gastroenteritise | 7 (8.3) | 1 (6.7) | 0 (0.0) | 21 (3.7) | 2 (8.0) | 0 (0.0) | 4 (2.8) |
| Herpes simplex infectionsf | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Acne | 4 (4.8) | 0 (0.0) | 0 (0.0) | 4 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Folliculitis | 2 (2.4) | 0 (0.0) | 0 (0.0) | 6 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 0 (0.0) | 1 (6.7) | 0 (0.0) | 6 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Source: Adapted from FDA Review
a Upper respiratory infections include nasopharyngitis, upper respiratory tract infection, pharyngitis, rhinitis, viral upper respiratory tract infection, tonsillitis, sinusitis, pharyngitis streptococcal, pharyngitis bacterial, peritonsillar abscess, viral rhinitis, and influenza
b Candida infections include oral candidiasis, oropharyngeal candidiasis, vulvovaginal candidiasis, vulvovaginal mycotic infection, oral fungal infection, oropharyngitis fungal, skin candida, genital candidiasis, and fungal pharyngitis
c Injection site reactions include injection site reaction, injection site erythema, injection site pain, injection site edema, injection site bruising, and injection site swelling
d Tinea infections include tinea pedis, fungal skin infection, tinea versicolor, tinea cruris, tinea infection, body tinea, and onychomycosis
e Gastroenteritis includes gastroenteritis, gastroenteritis viral, gastroenteritis bacterial, enterovirus infection, diarrhea, nausea, vomiting, enteritis, enterocolitis, and gastritis
f Herpes simplex infections include herpes simplex and oral herpes
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.