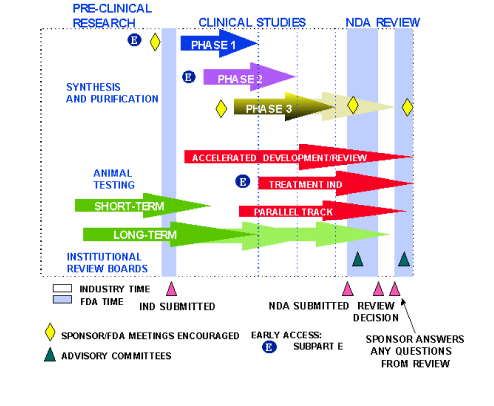
New Drug Development and Review Process
The mission of FDA's Center for Drug Evaluation and Research (CDER) is to assure that safe and effective drugs are available to the American people. This section has definitions and interactive charts which provide basic information for small business and others who are unfamiliar with the new drug development and approval process.
American consumers benefit from having access to the safest and most advanced pharmaceutical system in the world. The main consumer watchdog in this system is CDER. The center's best-known job is to evaluate new drugs before they can be sold. The center's evaluation not only prevents quackery, but also provides doctors and patients the information they need to use medicines wisely. CDER ensures that drugs, both brand-name and generic, work correctly and that their health benefits outweigh their known risks.
Drug companies seeking to sell a drug in the United States must first test it. The company then sends CDER the evidence from these tests to prove the drug is safe and effective for its intended use. A team of CDER physicians, statisticians, chemists, pharmacologists, and other scientists reviews the company's data and proposed labeling. If this independent and unbiased review establishes that a drug's health benefits outweigh its known risks, the drug is approved for sale. The center doesn't actually test drugs itself, although it does conduct limited research in the areas of drug quality, safety, and effectiveness standards.
Before a drug can be tested in people, the drug company or sponsor performs laboratory and animal tests to discover how the drug works and whether it's likely to be safe and work well in humans. Next, a series of tests in people is begun to determine whether the drug is safe when used to treat a disease and whether it provides a real health benefit.
The New Drug Development Process:
Steps from Test Tube to New Drug Application Review
- Overview
- How Drugs Are Developed: This web page provides an example on how a drug sponsor can work with FDA's regulations and guidance information to bring a new drug to market, from clinical trials to postmarketing surveillance.
- Overview of the Sequence of Drug Development Activities: PDUFA Activities in Drug Development
- Types of New Drug Applications
- Therapeutic Biologic Applications (BLAs)
- Combination Products Program: Combination products often involve cutting edge, novel technologies that raise not only unique scientific and technical questions, but also regulatory challenges related to where and how they should be regulated in order to ensure adequate and consistent regulatory oversight.
- New Drug Application Process
- Meetings with FDA
- New Drug Development Costs
- Statistics
- International Conference on Harmonization: The ICH is a cooperative effort between the drug regulatory authorities and the pharmaceutical company professional organizations in the European Union, Japan, and the United States to reduce the need to duplicate the testing conducted during the research and development of new drugs. This page provides a link to the ICH home page and ICH guidances. Please see the International Programs web page for FDA's work with ICH.