GUIDANCE DOCUMENT
CVM GFI #13 Evaluation of Effectiveness of New Animal Drugs for Use in Free-Choice Feeds-Medicated Block January 1985
- Docket Number:
- FDA-2021-D-0624
- Issued by:
-
Guidance Issuing OfficeCenter for Veterinary Medicine
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
PUBLIC HEALTH SERVICE
FOOD AND DRUG ADMINISTRATION
CENTER FOR VETERINARY MEDICINE
Guidelines for Evaluation of Effectiveness of New Animal Drugs for Use in Free-Choice Feeds revision of Medicated Block
Revised January 1985
Background:
This guideline is a revision of The Cattle Medicated Block Guideline. It is applicable to all free-choice feeds that may contain new animal drugs. The variant pattern of consumption of free-choice feeds (blocks, loose minerals, and liquid feeds) by ruminants (cattle and sheep) is well known. Intake enticement and control are necessary to provide a relatively steady quantity (amount and frequency) of medicated feed consumption.
When a drug is added to a free-choice feed, it is important that the target animal consumes enough of the drug for the drug to be effective, but does not consume an excessive amount of drug which might cause toxicity or violative drug residues. Since there is a large variation in consumption of free-choice feed, both intake control and effectiveness of the drug must be demonstrated when the drug is consumed on an irregular basis.
Feed composition (i.e., levels of salt, molasses, minerals or protein), physical form, and in some cases, drug content, regulate consumption and consequently dosage of a drug. Therefore, any change in feed composition, physical form, or drug content will require a supplemental approval of the specific free-choice feed.
New animal drugs must have been shown to be safe to the target animal, and edible products from treated animals must have been shown to be safe for humans before they can be considered under these guidelines. Though not a requirement, new animal drugs are normally approved for continuous use (e.g., feedlot) before they are approved for use in free-choice feeds. Thus, historical data are usually available for review before drugs are to be used in free-choice feeds. In the event there are no historical data on such a new animal drug, all aspects of Form FD 356 apply in developing the approval application.
Purpose:
The purpose of these guidelines is to assist drug and/or feed manufacturers in the development of data necessary for approval to market new animal drugs in free-choice feeds. These guidelines reflect current knowledge and are subject to revisions to accommodate new information and provide for circumstances now unforeseen.
Scope:
The guidelines are intended to:
- Assist in developing information and data needed to support Agency approval for the addition of production type new animal drugs to free-choice feeds.
- Clarify approval procedures for adding production type new animal drugs to free-choice feeds.
Efficacy Requirements and Data Analysis:
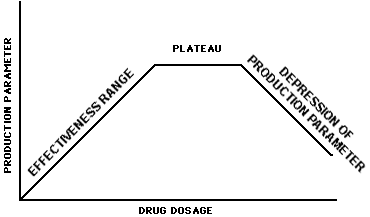
To determine the range of effectiveness of a drug in free-choice feeds, hand-fed pasture studies are recommended. Data should be collected for each claim/production class. These data may be collected in conventional pasture studies or in drylot studies (restricted to feed efficiency claims) where fresh pasture clippings and/or other feed are transported to the animals. The data are to represent three geographical locations in which the medicated free-choice feed will be marketed. Ideally, these studies should be designed to determine the dose-response characteristics of the drug including the "Effectiveness Range," "Plateau," and the "Depression of Production Parameter" areas as described in the following graph.
The "Effectiveness Range" is the levels of drug which give an increasing production response with increasing dosage. The "Plateau" is the part of the response curve beyond the "Effectiveness Range" that does not result in a change in production when the drug level is increased. The "Depression in Production Parameter" portion of the curve is beyond the "Plateau" and represents the area where a reduction in effectiveness occurs with an increase in dose.
The minimum data that must be available from the dose-response studies (hand feeding) are a description of the "Effectiveness Range." However, if available, the additional data dealing with the "Plateau" and "Depression of Production Parameter" will be useful to facilitate the decision-making process described in the next two sections of this guideline. For the purpose of this guideline, when the "Depression of Production Parameter" portion of the curve has not been determined, any drug level consumed above the highest level tested will be considered to be in the "Depression of Production Parameter" area of the dose-response curve. If the sponsor of the hand-feeding data desires an NADA approval for hand feeding the drug to pastured animals, all aspects of Form FD 356 apply in developing the new animal drug application. Reference is made to the "Research Model," "Definitions," and "Administrative Procedures" sections below.
Once the dose-response data are available, a sponsor of a medicated free-choice feed may obtain approval through either of the following approaches. The application for approval for each medicated free-choice feed must contain both efficacy and consumption data. Efficacy data may be collected for each medicated free-choice feed as described in "Approach I." The data must be specific for that particular approval. Alternately, efficacy data may come from a general medicated free-choice approval and be universally applicable as described in "Approach II."
Approach I:
A manufacturer may choose to pursue approval of a medicated free-choice feed for which free-choice efficacy data have not been collected. If dose-response data as described above are available, the feed manufacturer may collect efficacy and consumption data in at least one location and consumption data at a minimum of two additional locations within the proposed market area. The efficacy study must be sufficiently replicated to show the drug to be repeatedly effective in the free-choice feed. The consumption studies should approximate as close as possible the efficacy studies in number of animals, replications, duration, and Coefficients of Variation (CVs) are to be determined for both the efficacy study and the two consumption studies. If there are significant consumption differences among the locations and if the mean consumption for any location falls above the "Plateau" or below the minimum effective level (lowest point on the "Effectiveness Range"), the medicated free-choice feed is not approvable. The CVs of the consumption studies must be equal to or less than the CV of the efficacy study. The data generated in these studies apply only to the claim/production class and ruminant species involved, and these data may not be used to generate approval for any other free-choice feed. Reference is made to the "Research Model," "Definitions," and "Administrative Procedures" sections below.
Approach II:
Using the "Effectiveness Range" for the drug obtained in the hand-fed pasture studies, the drug will be tested in a free-choice feed (block, loose mineral, or liquid feed). Three studies in three geographical locations are to be conducted to determine drug efficacy and drug consumption in an appropriate free-choice environment.
It is important that the average drug consumption for the studies be within the "Effectiveness Range." The consumption data and the efficacy data are to be analyzed and a CV for the pooled studies determined for the treatment consumption data. This CV is a measure of random differences among the weekly observation periods after appropriate adjustments have been made for time, location, replicates, and animal numbers. It is important also to note that this CV will be determined only from those animals that consumed the medicated free-choice feed. The sponsor of these studies must request and obtain an approval to market the tested medicated free-choice feed. A regulation concerning use of the medicated feed must be published. These data may be referenced to support approval of an unlimited number of medicated free-choice feed applications for the specific claim/animal class if used in conjunction with appropriate consumption data as described below.
All other medicated free-choice feed manufacturers must simply collect consumption data under the conditions of the previously described efficacy/consumption studies. At least three studies are required, and they should represent the proposed market area of the medicated free-choice feed. A minimum of three replicates at each location should be used, and the design need not have unmedicated control groups. However, the consumption studies should approximate as close as possible the studies used to establish efficacy in terms of number of animals, replications, and duration; or the estimate of the CV obtained from these consumption studies must be adjusted for differences in design from the efficacy studies, particularly in numbers of animals, to make the two CVs comparable. It is important that the average drug consumption for the study be within the "Effectiveness Range." An appropriate pooled CV as described above is to be calculated for these consumption data. If there are significant consumption differences among the locations and if the mean consumption for any location falls above the "Plateau" or below the minimum effective level (lowest point on the "Effectiveness Range"), the medicated free-choice feed is not approvable.
The two CVs from the two groups of studies will be compared to determine if the data from the consumption studies are similar to the consumption data obtained in the efficacy trials. The CV of the consumption studies must be equal to or less than the CV of the consumption studies must be equal to or less than the CV for the efficacy studies.
Reference is made to the "Research Model," "Definitions," and "Administrative Procedures" sections below. All aspects of Form FD 356 apply in developing an application associated with the consumption data. Additionally, all aspects of Medicated Feed Application (FD 2800) apply.
Administrative Procedures:
Background: Depending on the amount of cooperation between the drug sponsor and the manufacturer of the free-choice feed, the collection of data needed for approval of an NADA involving a medicated free-choice feed may take either of the above approaches. Either the sponsor or manufacturer may generate any component of the data needed for a medicated free-choice approval; however, it is probable under "Approach II" that the hand-feeding and free-choice feed efficacy studies will be collected by the drug sponsor, while the consumption data is collected by the free-choice feed manufacturer.
Regardless of the data collection process employed, Agency approval will be for either an original NADA or a supplemental NADA. The data generated under the hand-feeding studies and under the efficacy studies with a medicated free-choice feed should result in NADA approvals for the applications(s) sponsor. The hand-feeding studies would provide for the use of the drug in hand-fed pasture situations, and the medicated free-choice feed efficacy data would provide for the use of the drug in the specific free-choice product for the claim/production class.
Process: To obtain approval to market a specific medicated free-choice feed, the applicant may use either of the following administrative approaches:
- File an NADA which contains dose-response data along with free-choice feed efficacy data and consumption data as described in "Approach II" plus information to satisfy all other parts of the Form FD 356.
- File an NADA which contains dose-response data along with efficacy and consumption data as described in "Approach I" plus information to satisfy all other parts of the Form FD 356.
- File a medicated feed application (FD 1800) to manufacture an approved specific medicated free-choice feed whose formula has been published in the FEDERAL REGISTER.
- Establish a Master File which contains consumption data as described in "Approach II" or the efficacy and consumption data as described in "Approach I" and which contains appropriate manufacturing and stability data as described in the Form FD 356. The sponsor of the Master File must authorize the Agency to reference the data in its Master File to support approval of a supplemental NADA (submitted by a drug sponsor who has generated the appropriate dose-response data and has generated efficacy data as described in "Approach II." The drug sponsor must request permission to market a medicated free-choice feed for the claim/production class used in the conduct of consumption and/or efficacy data. Approval of the supplement will not be published in the FEDERAL REGISTER because the approval will not affect or alter the content of the regulation. The approval will provide a basis for the medicated free-choice feed manufacturer to submit for Agency approval a medicated feed application (FD 1800) under section 512(m) of the act.
- Establish a master file which contains the efficacy and consumption data as described in "Approach I" and which contains appropriate manufacturing and stability data as described in the Form FD 356. The sponsor of the master file must authorize the Agency to reference the data in its master file to support approval of a supplemental NADA. The drug sponsor must request permission to market a medicated free-choice feed for the claim/production class used in the conduct of the consumption and efficacy data. Approval of the supplement will be published in the FEDERAL REGISTER and will provide a basis for a specific medicated free-choice feed manufacturer to submit for Agency approval a medicated feed application (FD 1800) under section 512(m) of the act for the tested formulation.
Research Model:
- Number of Animals: At least eight (8) per pasture replicate.
- Number of Replicates: At least three (3) per treatment.
- Number of Locations: At least three (3) within the proposed market area.
- Duration: At least 91 days or other length that emulates production claim.
- Animal Sex : Must be representative of the target production class.
- Animal Breed: Must be representative of the target production class.
- Environmental Effect: The drug containing free-choice feed must be exposed to the environment and evaluated (weight/chemical) periodically to monitor environmental losses. The environmental effect must be determined with the same frequency as the consumption weighbacks are measured.
- Season of Year: Studies must be during the period(s) of proposed use of the free-choice feed.
- Indication for Use: Each specific claim/production class must have an independent data base.
- Supplemental Feed: Weekly records must be maintained on all supplemental feed. Treatment and control animals must receive similar supplemental feed.
- Medication: Records must be made on all medical treatment received by the animal.
- Death: The cause of death for all animals in the study should be determined by necropsy.
- Drug Consumption: Medicated free-choice feed must be available at all times and consumption determined weekly (each seven days) with feed weighbacks (orts) adjusted for environmental losses (item 7 above).
- Pasture Conditions: The amount, quality, and type of feed available in the test pastures should be described on a weekly basis such that appropriate conditions of use can be stated in the labeling.
Definitions:
- Animal Production Classes: Preweaned (suckling), weaned (stocker/feeder), and mature animals (breeding).
- Coefficient of Variation: Standard deviation expressed as a percent of the mean from an appropriate statistical model.
- Consumption Study: An experiment in which voluntary consumption of a feed containing the drug is determined over an appropriate time period. To be valid, consumption studies must closely approximate the appropriate efficacy studies in number of animals used, number of replications, and duration.
- Depression of Production Parameter: That part of the dose-response curve beyond the "Plateau" where increasing levels of drug result in a decrease in response of the production parameter.
- Drug Sponsor: Manufacturer and/or distributor of the drug.
- Effectiveness Range: That section of the dose-response curve in which increasing levels of drug result in increasing responses of the production parameter.
- Efficacy Data: An experiment in which the amount of drug voluntarily eaten by the animal is measured as well as the animal's response (weight gain, feed efficiency, milk production, etc.). Treatment groups must include unmedicated controls.
- Feed: Article of food for animals as defined in 201(f) of the act.
- Free-Choice Environment: Types of animal accommodations that dictate the amount of feed provided directly to the animals. Feedlot and drylot represent one type environment where all or practically all of the feed is provided directly to the animals. Range and pasture environments imply that the animals graze or browse for the major portion of their feed with the remainder provided by supplemental feeding.
- Free-Choice Feed: More than one day's supply of feed offered to animals under conditions where consumption is voluntary (this food is never intended to be the only source of nutrients available for the animal), e.g., blocks, loose minerals, and liquid feeds. The formula for this feed is specific and is described in the New Animal Drug Application or in the Master File.
- Full Feed: Providing feed to animals which is intended to be the only source of nutrients (feeding animals in feedlots or in drylots).
- Geographical Location: Sections within a market area that differ in management practices, feed availability, climate, etc., which may affect feed consumption and/or drug response.
- Market Area: Geographical area in which the medicated free-choice feed is sold.
- Medicated Feed: Animal feed that contains a drug regulated under 21 CFR 500.
- Plateau: That part of a dose-response curve beyond the "Effectiveness Range" where increasing levels of drug do not result in a change in the production parameter.
- New Animal Drug: Any substance conforming to section 201(w) of the act and published in 21 CFR 500-599.
- Replicate: An independent experimental unit to which the same treatment is applied.
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5))
If unable to submit comments online, please mail written comments to:
Dockets Management
Food and Drug Administration
5630 Fishers Lane, Rm 1061
Rockville, MD 20852
All written comments should be identified with this document's docket number: FDA-2021-D-0624.
Questions?
- CVM
- Center for Veterinary Medicine
Food and Drug Administration
5001 Campus Drive
College Park, MD 20740
- AskCVM@fda.hhs.gov
- (240) 402-7002