White Oak Campus Information
Site Address: 10903 New Hampshire Avenue, Silver Spring, MD 20993
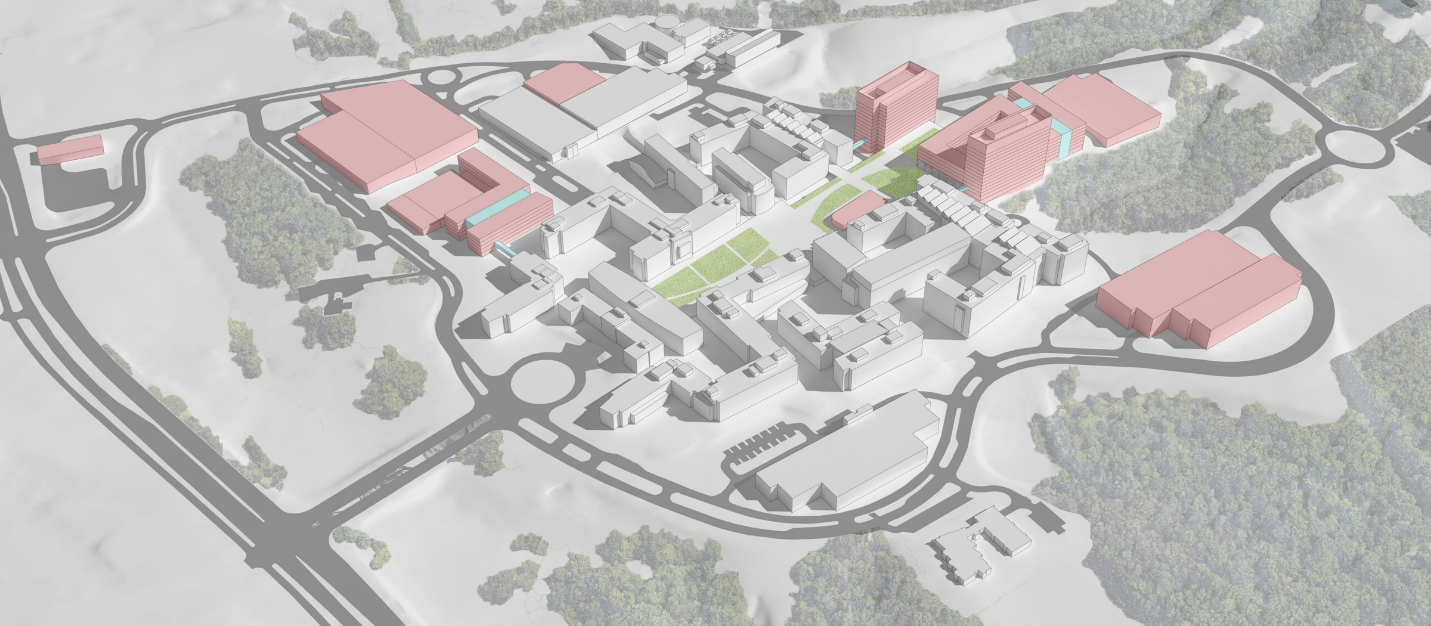
Currently, the FDA White Oak Campus consists of ten office and four laboratory buildings, totaling 3.1 million rentable square feet. The Campus, located on GSA’s Federal Research Center (FRC) in Silver Spring, MD, was authorized by the FDA Revitalization Act of 1990 and construction was funded through GSA appropriations. Subsequent groundbreaking legislation, including the Family Smoking Prevention and Tobacco Control Act of 2009, the FDA Food Safety Modernization Act of 2011, the FDA Safety and Innovation Act of 2012, the Drug Quality and Security Act of 2013, and the 21st Century Cures Act of 2016, expanded FDA’s responsibilities. Additionally, the FDA Reauthorization Act of 2017 ensured user-fee funding to continue expanding FDA’s workforce through fiscal year 2022. Consequently, FDA partnered with GSA for developing a new FRC Master Plan to expand the existing Campus, which the National Capital Planning Commission approved in December 2018. The Master Plan’s features remain consistent with the original intent of the FDA consolidation on the White Oak Campus, to collocate FDA’s headquarters programs to promote operational and scientific excellence in support of FDA’s strategic priorities. Funding for and timing of additional Campus construction envisioned in the Master Plan is dependent on GSA appropriations.