Tobacco Retailer Warning Letters - Overview
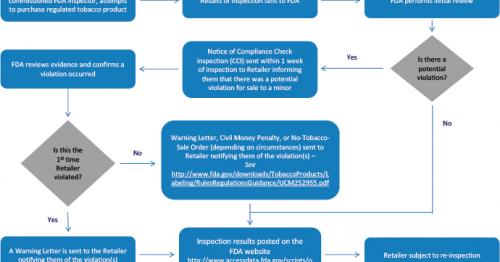
We generally send warning letters to retailers the first time a tobacco compliance check inspection reveals a violation of the federal tobacco laws and regulations that FDA enforces.
Click for full version of
Undercover Buy Inspections
During Undercover Buy Inspections, the retailer is unaware an inspection is taking place. The underage purchaser and inspector will not identify themselves.
Failure to promptly and adequately correct all violations and ensure compliance with all applicable laws and regulations may lead to enforcement actions, including Civil Money Penalties or No-Tobacco-Sale Orders.
We issue warning letters to traditional “brick and mortar” retail stores nationwide and online retailers.
What should you do if you receive a Warning Letter?
- Review the letter carefully to see what charges are listed.
- Respond to the Warning Letter within 15 working days, in writing, by mail or email. Include the following in your response to the Warning Letter:
- An explanation of the steps you will take to correct the violation(s) and prevent future violations (for example, retrain your employees, remove the problematic items, etc.); and
- Your current contact information including telephone number and email address.
- Promptly and adequately correct the violations listed and be sure that you comply with all applicable laws and regulations.
- If you have any questions about warning letters, contact the Center for Tobacco Products at 1-877-CTP-1373 or via email to the address listed in your warning letter: CTP-Compliance-WL-Correspondence@fda.hhs.gov or CTPCompliance@fda.hhs.gov.
The Warning Letter includes:
- References to relevant laws and regulations
- The date the store was inspected, and if a sale was made, the approximate time
- The particular violation(s) inspectors observed and an explanation of the evidence used to support the violation(s)
- A statement directing the retailer to correct the violation(s)
- Notice that enforcement action may come without further notice if violations are observed in the future
- A request to submit a written response to FDA within (15) working days of receiving the Warning Letter
FDA Age Calculator
Download the “FDA Age Calculator,” a voluntary smartphone application to help retailers comply with federal, state, and local age restrictions for selling tobacco products.