Implantable System for Remodulin - P140032
This is a brief overview of information related to FDA's approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA's approval.
Product Name: Implantable System for Remodulin
PMA Applicant: Medtronic
Address: 8200 Coral Sea Street NE, MS-MVS11, Mounds View, MN 55112
Approval Date: December 22, 2017
Approval Letter: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140032A.pdf
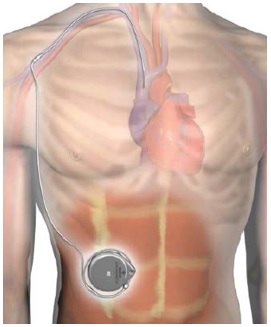
What is it? The Implantable System for Remodulin is an infusion system that is fully implanted into a patient to deliver Remodulin through a patient's veins. Remodulin (or Treprostinil) is a medication used to treat pulmonary arterial hypertension (high blood pressure in the arteries that go from the heart to the lungs).
The Implantable System for Remodulin is made up of the following parts:
- Medtronic SynchroMed II 8637P Programmable Pump (the "pump")
- Medtronic N'Vision 8840 Clinician Programmer with 8870 Application Card (the programmer")
- Medtronic 8201 Implantable 80 cm Intravascular Catheter (the "catheter")
How does it work? Surgeons insert the intravascular catheter through a vein at the superior caval-atrial junction (the joint between the superior vena cava and the heart) and connect the catheter to the pump in a pump pocket placed beneath the abdominal skin. The surgeon then uses the handheld programmer device to program and review the pump's settings. Once the surgeon programs the pump, the Implantable System for Remodulin delivers the remodulin injection from the pump reservoir, through the pump tubing, the catheter port, and the catheter to the intravascular delivery site. The pump remains permanently implanted and the health care provider uses a needle and syringe refill kit to refill the pump with Remodulin as needed.
When is it used? The Implantable System for Remodulin is used in adult patients with New York Heart Association (NYHA) Class I, II and III pulmonary arterial hypertension who need intravenous delivery of Remodulin.
What will it accomplish? The Implantable System for Remodulin delivers a continuous infusion of Remodulin through a patient's veins to stop narrowing of the pulmonary arteries, help supply blood to the lungs, and keep a patient's blood pressure within a healthy range.
When should it not be used? The Implantable System for Remodulin should not be used in the following patients:
- NYHA Class IV heart failure patients.
- Patients who cannot tolerate a sudden cessation of Remodulin therapy.
- Patients with a known or suspected infection, bacteremia, or sepsis requiring antibiotics.
- Patients with vasculature that is inadequate for an 8 French introducer or catheter advancement without stylet guidance.
- Patients implanted with leads or catheters (active or abandoned) in the superior vena cava that cannot be removed prior to or at system implant.
- Patients whose body size is not sufficient to accept pump bulk and weight.
- Patients with skin or soft tissue that would heal poorly, increase susceptibility to infections, or is unacceptable for implant of this system.
- Patients for which a health care provider cannot implant the pump 2.5 cm or less from the surface of the skin.
Additional information (including warnings, precautions, and adverse events): Summary of Safety and Effectiveness Data and labeling are available online.