Public Notification: Linsen Double Caulis Plus (靈仙双藤素) Contains Hidden Drug Ingredient
[10-30-2017] The Food and Drug Administration (FDA) is advising consumers not to purchase or use Linsen Double Caulis Plus (靈仙双藤素), a product promoted for joint, gout, and back pain on various websites, including http://www.tranpharma.com, and possibly in some retail stores.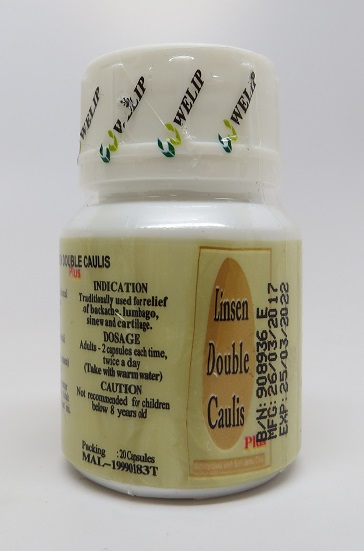

FDA laboratory analysis confirmed that Linsen Double Caulis Plus (靈仙双藤素) contains dexamethasone. Dexamethasone is a corticosteroid commonly used to treat inflammatory conditions. Corticosteroid use can impair a person’s ability to fight infections and can cause high blood sugar levels, muscle injuries and psychiatric problems. When corticosteroids are taken for a prolonged period, or at high doses, they can suppress the adrenal gland. Abrupt discontinuation can cause withdrawal symptoms.
Consumers taking Linsen Double Caulis Plus (靈仙双藤素) should immediately consult with their health care provider to safely discontinue use of this product. A health care professional should assess the risks of withdrawal from corticosteroids.. Only licensed health care professionals can evaluate patients for the risk or existence of adrenal suppression. To date, FDA is not aware of any reports of adverse events related to this product.
In addition, the undeclared ingredient in Linsen Double Caulis Plus (靈仙双藤素) may cause serious side effects when combined with other medications.
Health care professionals and patients are encouraged to report adverse events or side effects related to the use of this product to FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report online MedWatch Online Voluntary Reporting Form, or:
- Download and complete the form, then submit it via fax at 1-800-FDA-0178.
Note: This notification is to inform the public of a growing trend of dietary supplements or conventional foods with hidden drugs and chemicals. These products are typically promoted for sexual enhancement, weight loss, and body building and are often represented as being “all natural.” FDA is unable to test and identify all products marketed as dietary supplements that have potentially harmful hidden ingredients. Consumers should exercise caution before purchasing any product in the above categories.
For more information:

