Drugs@FDA Data Files
Below you will find a compressed data file of the Drugs@FDA database. It does not include the scripts (programming) we use to produce the online version of Drugs@FDA. We are providing this technical information for users who are familiar with working with databases or spreadsheets. All fields are separated by tab delimiters. Each table's primary key, data types, field lengths and nulls appear in the list below. While the official online application, Drugs@FDA, is updated daily, this data file is updated once per week, on Tuesday.
Once you have downloaded the compressed file (drugsatfda.zip), you can unzip the file into 11 text tables. You can then import the tables into a database, spreadsheet or word processing program. Generally, a database program is the best program to use for these types of files.
We cannot offer guidance on how to construct your database design, as each user has different requirements or uses.
Download File
- Drugs@FDA Download File (ZIP - 3.94 MB)
Data Last Updated: April 16, 2024
Data Definitions and ERD for Drugs@FDA (as of Nov. 2, 2016)
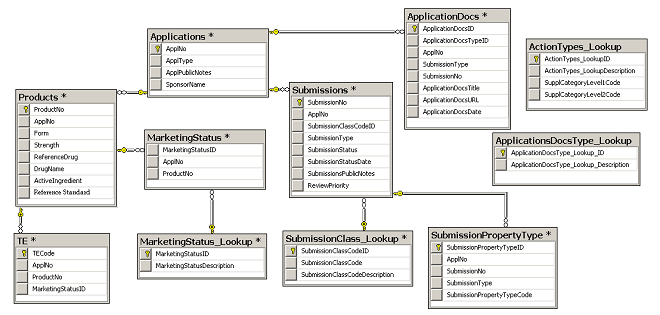
Entity Relationship Diagram
Drugs@FDA consists of 11 tables:
ActionTypes_Lookup
- [ActionTypes_LookupID] [int] IDENTITY(1,1) NOT NULL
- [ActionTypes_LookupDescription] [varchar](100) NOT NULL
- [SupplCategoryLevel1Code] [varchar](100) NULL
- [SupplCategoryLevel2Code] [varchar](100) NULL
ApplicationDocs
- [ApplicationDocsID] [int] IDENTITY(1,1) NOT NULL
- [ApplicationDocsTypeID] [int] NOT NULL
- [ApplNo] [char](6) NOT NULL
- [SubmissionType] [char](10) NOT NULL
- [SubmissionNo] [int] NOT NULL
- [ApplicationDocsTitle] [varchar](100) NULL
- [ApplicationDocsURL] [varchar](200) NULL
- [ApplicationDocsDate] [datetime] NULL
Applications
- [ApplNo] [char](6) NOT NULL
- [ApplType] [char](5) NOT NULL
- [ApplPublicNotes] [text] NULL
- [SponsorName] [char](500) NULL
ApplicationsDocsType_Lookup
- [ApplicationDocsType_Lookup_ID] [int] IDENTITY(1,1) NOT NULL
- ApplicationDocsType_Lookup_Description] [varchar](200) NOT NULL
MarketingStatus
- [ApplNo] [char](6) NOT NULL,
- [ProductNo] [char](3) NOT NULL,
- [MarketingStatusID] [int] NOT NULL
MarketingStatus_Lookup
- [MarketingStatusID] [int] IDENTITY(1,1) NOT NULL
- [MarketingStatusDescription] [varchar](200) NOT NULL
Products
- [ApplNo] [char](6) NOT NULL
- [ProductNo] [char](6) NOT NULL
- [Form] [varchar](255) NULL
- [Strength] varchar](240) NULL
- [ReferenceDrug] [int] NULL
- [DrugName] [varchar](125) NULL
- [ActiveIngredient] [varchar](255) NULL
- [ReferenceStandard] [int] NULL
SubmissionClass_Lookup
- [SubmissionClassCodeID] [int] IDENTITY(1,1) NOT NULL
- [SubmissionClassCode] [varchar](50) NOT NULL
- [SubmissionClassCodeDescription] [varchar](500) NULL
SubmissionPropertyType
- [ApplNo] [char](6) NOT NULL
- [SubmissionType] [char](10) NOT NULL
- [SubmissionNo] [int] NOT NULL
- [SubmissionPropertyTypeCode] [varchar](50) NOT NULL (Orphan or NULL)
- SubmissionPropertyTypeID [int] NOT NULL
Submissions
- [ApplNo] [char](6) NOT NULL
- [SubmissionClassCodeID] [int] NULL
- [SubmissionType] [char](10) NOT NULL
- [SubmissionNo] [int] NOT NULL
- [SubmissionStatus] [char](2) NULL
- [SubmissionStatusDate] [datetime] NULL
- [SubmissionsPublicNotes] [text] NULL
- [ReviewPriority] [varchar](20) (Standard, Priority, NULL)
TE
- [ApplNo] [char](6) NOT NULL
- [ProductNo] [char](3) NOT NULL
- [MarketingStatusID] [int] NOT NULL
- [TECode] [varchar](100) NOT NULL