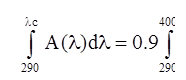SMALL ENTITY COMPLIANCE GUIDE
Labeling and Effectiveness Testing: Sunscreen Drug Products for Over-The-Counter Human Use — Small Entity Compliance Guide Guidance for Industry December 2012
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
December 2012
OTC
Guidance for Industry
Labeling and Effectiveness Testing: Sunscreen Drug Products for Over-The-Counter Human Use — Small Entity Compliance Guide
Additional copies are available from:
Office of Communications, Division of Drug Information
Center for Drug Evaluation and Research
Food and Drug Administration
10903 New Hampshire Ave., Bldg. 51, rm. 2201
Silver Spring, MD 20993-0002
Tel: 301-796-3400; Fax: 301-847-8714; E-mail: druginfo@fda.hhs.gov
http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
December 2012
OTC
TABLE OF CONTENTS
I. INTRODUCTION
II. SUMMARY OF THE REGULATION AND THIS GUIDANCE
III. QUESTIONS AND ANSWERS
Guidance for Industry1
Labeling and Effectiveness Testing: Sunscreen
Drug Products for Over-the-Counter Human Use —
Small Entity Compliance Guide
I. INTRODUCTION
This guidance is intended to help small businesses understand and comply with the Food and Drug Administration’s (FDA’s) labeling and testing regulations for certain over-the-counter (OTC) sunscreen drug products. On June 17, 2011, we published the final rule “Labeling and Effectiveness Testing: Sunscreen Drug Products for Over-the-Counter Human Use” (2011 sunscreen final rule), 76 FR 35620. Drug products subject to the 2011sunscreen final rule must bear specific labeling to promote their safe and effective use. The 2011 sunscreen final rule also requires that these drug products be tested according to specific testing procedures that ensure product effectiveness. On May 11, 2012, we published a 6-month delay of the compliance dates in the 2011 sunscreen final rule, 77 FR 27591. This guidance was prepared in accordance with section 212 of the Small Business Regulatory Enforcement Fairness Act (Public Law 104-121).
FDA’s guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency’s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
II. SUMMARY OF THE REGULATION AND THIS GUIDANCE
In the Federal Register of August 27, 2007 (72 FR 49070), we published a proposed rule that included labeling and testing for OTC sunscreen drug products. The rule proposed labeling and testing requirements for ultraviolet A radiation (UVA) and ultraviolet B radiation (UVB) protection. After reviewing information submitted in response to the proposed rule and other pertinent information, we published the 2011 sunscreen final rule, which is codified in FDA regulations as 21 CFR 201.327. Manufacturers of drug products with annual sales less than $25,000 must comply with the final rule by December 17, 2013. The compliance date for all other drug products subject to the final rule is December 17, 2012.2
The 2011 sunscreen final rule applies to OTC sunscreen drug products that are marketed without an approved new drug application (NDA) or abbreviated new drug application (ANDA) and that contain any of the ingredients described in section III., Questions and Answers, Question 1. The rule prescribes labeling required to be used on the drug products’ principal display panel and Drug Facts labeling, and describes the methods for conducting two effectiveness test procedures required to be able to make certain claims, namely the sun protection factor (SPF) Test (21 CFR 201.327(i)) and the Broad Spectrum Test (21 CFR 201.327(j)). The SPF Test provides a clinical (in vivo) measurement of a sunscreen drug product’s ability to protect against sunburn (which is caused primarily, but not entirely, by UVB). 3 The Broad Spectrum Test provides an in vitro measurement of a sunscreen drug product’s ability to protect against both UVA and UVB radiation. These requirements and corresponding claims are described in greater detail in section III., Questions and Answers, in this guidance. Question 1 clarifies which drug products are subject to the 2011 sunscreen final rule, Questions 2 through 5 address labeling concerns, Questions 6 through 14 pertain to efficacy testing, and Questions 15 through 17 are about other aspects of the implementation of the 2011 sunscreen final rule.
III. QUESTIONS AND ANSWERS
Question 1: What drug products are subject to the 2011 sunscreen final rule?
Answer: The 2011 sunscreen final rule applies to OTC sunscreen drug products that:
- Are marketed without approved drug applications (without NDAs or ANDAs) and
- Contain any of the following active ingredients, alone or in combination: aminobenzoic acid (PABA), avobenzone, cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, oxybenzone, padimate O, sulisobenzone, titanium dioxide, trolamine salicylate, zinc oxide
The 2011 sunscreen final rule does not apply to OTC sunscreen drug products that are marketed under an approved NDA or ANDA. Note that sunscreens containing any active ingredients, alone or in combination, that are not described in 21 CFR 352.10 and 352.20, the sunscreen monograph that is presently stayed, currently would require an approved NDA or approved ANDA to be marketed.
Question 2: What are the labeling requirements for OTC sunscreen drug products subject to the 2011 sunscreen final rule?
Answer: Table 1 summarizes labeling requirements specific to OTC sunscreen drug products subject to the 2011 sunscreen final rule. In addition, OTC sunscreen drug products subject to this rule must comply with labeling requirements in 21 CFR part 201 that apply to all OTC drug products, including the Drug Facts requirements of 21 CFR 201.66. Additional directions and warnings that are applicable to particular drug products also should be included.
Table 1. Summary of Specific Labeling Requirements for OTC Sunscreen Drug Products Subject to the 2011 Sunscreen Final Rule
| Labeling Section | Labeling Required |
|---|---|
|
Principal Display Panel |
All sunscreen drug products must bear the statement of identity “sunscreen.” For sunscreen drug products that pass the Broad Spectrum Test in 21 CFR 201.327(j): For sunscreen drug products that do not pass the Broad Spectrum Test in 21 CFR 201.327(j): For sunscreen drug products that provide 40 or 80 minutes of water resistance according to the test in 21 CFR 201.327(i)(7): |
|
Uses
|
For all sunscreen drug products: For sunscreen drug products that are Broad Spectrum with SPF 15 or higher according to the tests in 21 CFR 201.327(i) and (j), use of the following additional statement is optional: |
|
Warnings |
For all sunscreen drug products: For sunscreen drug products that do not pass the Broad Spectrum Test or that have SPF values less than 15, the first statement under Warnings must be: |
|
Directions |
For all sunscreen drug products: “children under 6 months of age: Ask a doctor” Additional optional statements: “For sunscreen use” “apply to all skin exposed to the sun” For sunscreen drug products that are Broad Spectrum with SPF 15 or higher according to the tests in 21 CFR 201.327(i) and (j):
· limit time in the sun, especially from 10 a.m. - 2 p.m. For sunscreen drug products that do not satisfy the water-resistance test in 21 CFR 201.327(i)(7): For sunscreen drug products that satisfy the water-resistance test in 21 CFR 201.327(i)(7): |
|
Other Information |
“protect the product in this container from excessive heat and direct sun” |
Question 3: Does my new labeling need to be reviewed or approved by the FDA before I can use it on marketed products?
Answer: No. New labeling that complies with 21 CFR 201.327 as summarized above does not require premarketing review or approval by the FDA.
Question 4: Must I use the exact wording and format described in the rule?
Answer: In most cases, information shown in quotation marks in 21 CFR 201.327 and above must be used exactly as indicated. For statements under “Uses,” other truthful and nonmisleading statements describing only the uses that are described in 21 CFR 201.327(c) may be used, subject to the requirements of the Federal Food, Drug, and Cosmetic Act (FD&C Act) regarding misbranding and prohibiting the introduction or delivery for introduction into interstate commerce of unapproved new drugs. To ensure that your statement of “Uses” satisfies these requirements, we strongly encourage you to use the exact language provided in 21 CFR 201.327(c).
- As noted, additional labeling also may be required for individual drug products, and OTC sunscreens must also follow the Drug Facts labeling regulation, 21 CFR 201.66.
Question 5: Do I ever need to submit my labeling to the FDA?
Answer: Yes. Like other drugs, labeling of OTC sunscreen drug products subject to the 2011 sunscreen final rule must be submitted to the FDA as required by the drug listing provisions of section 510(j) of the FD&C Act and the FDA’s drug listing regulations in 21 CFR part 207. After initial listing of a drug and submission of its labeling, any labeling changes or updates must be submitted every June and December (section 510(j)(2) of the FD&C Act; 21 CFR 207.21(b)). However, registrants (or, if applicable, private label distributors) are encouraged to submit updates through the electronic registration and listing system more frequently, as changes occur. Information on this process can be found at Electronic Drug Registration and Listing Instructions.
Question 6: What are the solar simulator specifications for sunscreen testing and where can I find them?
Answer: The 2011 sunscreen final rule provides solar simulator specifications in 21 CFR 201.327(i)(1). Please review that regulation for the detailed requirements. In brief, that provision includes the following:
- Both single port and multiport solar simulators are allowed
- Solar simulators must be filtered to provide a continuous emission spectrum from 290 to 400 nanometers (nm) with a total irradiance limit of 1500 W/m2 for all wavelengths between 250 and 1400 nm
- Relative cumulative erythemal effectiveness range specifications are defined for each wavelength band
- UVA II (320 to 340 nm) and UVA I (340 to 400 nm) irradiance must equal or exceed 20 percent and 60 percent, respectively, of the total UV (290 to 400 nm) irradiance
- Regular calibration of solar simulators must occur at least once a year
- A 20 percent beam uniformity is required
Question 7: What is the sunscreen standard for use in the SPF Test?
Answer: 21 CFR 201.327(i)(2) requires the use of a 7-percent padimate O and 3-percent oxybenzone sunscreen standard formulation in the SPF test.
Question 8: What are the requirements for number of test subjects in the SPF Test?
Answer: We require valid test results in a minimum of 10 subjects (21 CFR 201.327(i)(3)). A maximum of three subjects may be rejected from the test panel.
Question 9: What sites on the body must be tested and what size must the test sites and subsites be for the SPF Test?
Answer: As stated in 21 CFR 201.327(i)(4)(i) and (ii), the test site must be on a subject’s back and the size of the test site must be at least 30 cm2. Test subsites must be at least 0.5 cm2 and must be separated by a distance of at least 0.8 cm.
Question 10: What are the requirements for applying sunscreen to test subjects for the SPF Test?
Answer: 21 CFR 201.327(i)(4)(iii) and (iv) require the following:
- Apply sunscreen using a finger cot to spread as evenly as possible. Presaturation of the finger cot (i.e., soaking the finger cot in the sunscreen before application) is not required.
- Apply sunscreen in a specified amount: 2 mg/cm2.
- Wait at least 15 minutes after applying the sunscreen before UV exposure.
Question 11: What do I need to do if I wish to make water-resistance claims in the labeling of my sunscreen drug product?
Answer: If you want to make water-resistance claims on your labeling, the water-resistance test in 21 CFR 201.327(i)(7) is required. The water-resistance test indicates that a sunscreen drug product’s labeled SPF protection is retained following immersion in water for a certain period of time (either 40 or 80 minutes).
The water-resistance component of the SPF Test consists of alternating water-immersion and drying procedures. The water-immersion procedure immediately follows the sunscreen application step in the SPF Test method. After sunscreen application, subjects are immersed in water to cover the test area for 20 minutes and immersion is followed by a 15-minute drying period. Then subjects are immersed again for 20 minutes followed by another 15-minute drying period. Now subjects are ready to be tested according to the remaining steps in the SPF Test method. This sequence must be performed to substantiate a “water-resistant (40 minutes)” claim.
To obtain a “water-resistant (80 minutes)” claim, the immersion and drying cycles must be repeated for a total of 4 immersion-drying sequences. After all the water-immersion and drying steps are completed, the SPF Test method resumes as it normally would. Consequently, the resulting SPF value represents the SPF protection retained after 80 minutes of water immersion.
Question 12: What is the Broad Spectrum Test and where can I find its specifications?
Answer: The Broad Spectrum Test is a test that measures a sunscreen drug product’s transmittance/absorbance of ultraviolet (UV) radiation across both the UVB and UVA regions of the spectrum. The specifications for this test method are found in 21 CFR 201.327(j). See that regulation for specific details. Table 2 briefly summarizes key test parameters.
Table 2. Key Requirements of the Broad Spectrum Test
| Test Parameter | Requirement |
|---|---|
|
Plate |
PMMA* plate (21 CFR 201.327(j)(1)(i)) |
|
Sample holder |
Holds the PMMA plate (roughened side up) in a horizontal position and is mounted as close as possible to the input optics of the spectrometer (21 CFR 201.327(j)(1)(ii)) |
|
Light source for transmittance measurements |
“produce a continuous spectral distribution of UV radiation from 290 to 400 nanometers” (21 CFR 201.327(j)(1)(iii)) |
|
Input optics: Bandwidth |
≤ 1 nanometer (21 CFR 201.327(j)(1)(iv)) |
|
Dynamic range of the spectrometer |
“sufficient to measure transmittance accurately through highly absorbing sunscreen” at all wavelengths between 290 nm and 400 nm (21 CFR 201.327(j)(1)(v)) |
|
Application of sunscreen drug product to plate |
0.75 mg/cm2 with two-phase spreading (21 CFR 201.327(j)(2)) |
|
Pre-irradiation dose |
Fixed at 800 J/m2-eff (21 CFR 201.327(j)(3)) |
|
Number of transmittance measurements |
At least five measurements of mean transmittance on three different plates (21 CFR 201.327(j)(4) and (6)) |
* Polymethylmethacrylate
Question 13: Is an in vivo test required to show Broad Spectrum protection?
Answer: No. We have concluded that the in vitro critical wavelength method provides an adequate measure of broad spectrum protection (21 CFR 201.327(j)). We do not require an in vivo test to demonstrate broad spectrum protection.
Question 14: What must I show to label a product as providing “Broad Spectrum” protection under this rule?
Answer: To label your sunscreen drug product as “Broad Spectrum,” you must conduct the in vitro test described in 21 CFR 201.327(j) and demonstrate that your drug product has a critical wavelength of at least 370 nm. The critical wavelength is the wavelength at which the area under the absorbance curve represents 90 percent of the total area under the curve in the UV region. The critical wavelength (λc) is expressed mathematically as:
In this equation, A(λ) is the mean absorbance at each wavelength, and dλ is the wavelength interval between measurements.
Question 15: When must I be in compliance with the 2011 sunscreen final rule?
Answer: The compliance date for all drug products subject to the rule with annual sales of $25,000 or more is December 17, 2012. For drug products subject to the rule with annual sales of less than $25,000, the compliance date is December 17, 2013.4
Question 16: What will happen if I fail to comply with the 2011 sunscreen final rule by the compliance dates?
Answer: If you fail to comply with the 2011 sunscreen final rule by the compliance dates, your drug product may be subject to regulatory action.
Question 17: If I have questions about whether my drug product is subject to the 2011 sunscreen final rule, how to comply with the 2011 sunscreen final rule, or any related issues, whom should I contact at the FDA?
Answer: You should contact the Division of Nonprescription Regulation Development. Contact information is available at Regulation of Nonprescription Products
This guidance has been prepared by the Division of Nonprescription Regulation Development, Office of Drug Evaluation IV, in the Center for Drug Evaluation and Research at the Food and Drug Administration.
These dates reflect the 6-month extensions to the compliance dates that were announced in a final rule published May 11, 2012 (77 FR 27591).
UVA also makes a contribution to sunburn. For “Broad Spectrum” sunscreens, the SPF also generally serves as a relative measure of the magnitude of Broad Spectrum protection. (See 76 FR 35620 at 35625.)
See 77 FR 27591 (May 11, 2012) and Public Law 112-144, section 1130.
Submit Comments
Submit comments on this guidance document electronically via docket ID: FDA-2013-S-0610 - Specific Electronic Submissions Intended For FDA's Dockets Management Staff (i.e., Citizen Petitions, Draft Proposed Guidance Documents, Variances, and other administrative record submissions)
If unable to submit comments online, please mail written comments to:
Dockets Management
Food and Drug Administration
5630 Fishers Lane, Rm 1061
Rockville, MD 20852
All comments should be identified with the title of the guidance.