Statistical Review and Evaluation - Evicel
DEPARTMENT OF HEALTH AND HUMAN SERVICES
PUBLIC HEALTH SERVICE
FOOD AND DRUG ADMINISTRATION
CENTER FOR BIOLOGICS EVALUATION AND RESEARCH
Division of Biostatistics (HFM-215)
STATISTICAL REVIEW AND EVALUATION
Date Populated: Jan. 14, 2008
Type/Application ID/Amendment #: BLA 125010/105
Proposed Use (Indication): Adjunct to hemostasis
Sponsor: Omrix.
Product name: EVICEL fibrin sealant (Human)
From: Chinying Wang, Ph. D. (HFM-219)
Through: Ghanshyam Gupta, Ph. D.
To: Debbie Cordaro (HFM-380)
Cc: Ghanshyam Gupta, Ph. D., Henry Hsu, Ph. D.,
Chron. File (HFM -215)
The study protocol entitled "A Prospective, Randomized Controlled Study to Compare the Effects of a Fibrin Sealant (FS2) as an adjunct to hemostasis for soft tissue bleeding during retroperitoneal or intra-abdominal surgery" is a phase III study comparing FS2 to Surgicel. This efficacy supplement contains study reports in support of a proposed new indication for adjunct to hemostasis in patients undergoing non-emergent retroperitoneal or intra-abdominal surgery procedures where a topical hemostat is indicated.
Statistical Review
The efficacy analysis for the primary endpoint has been reviewed. Efficacy was defined as FS2 was non-inferior to Surgical. The primary efficacy endpoint is defined as an absence of bleeding and no treatment with any hemostatic measures (other than the assigned hemostatic adjunct, FS2 or Surgicel) at 10 minutes following randomization to treatment. The analysis of primary endpoint is confirmed.
For the analysis of primary endpoint, the sponsor used a two-sided 95% confidence interval (CI) for the ratio of proportions of success. In the statistical analysis plan, it states that in case that the lower limit of the 95% CI is greater than 1.0, then there is evidence of superiority. Statistically, the procedure is agreeable. However, for the labeling of superiority of FS2 to Surgical, there is a concern for the conservativeness due to the original study design of non-inferiority. Using Fisher exact test (shown in the appendix), there is statistical difference between FS2 and Surgicel. The superiority claim would be fine.
In addition, analyses of secondary endpoints for absence of bleeding at 4 and 7 minutes following randomization have been performed. The survival analysis, using Kaplan-Meier curves was performed for time to hemostasis. The survival curves for FS2 and Surgicel are slightly different from that presented by the sponsor because the sponsor considered treatment failure as censored at 10minutes. The analyses are attached in the Appendix.
APPENDIX
(Ia) Contingency Analysis of SUCDC By TREATN (ITT primary endpoint)
TREATN By SUCDC
| Count Total % Col % Row % |
No |
Yes |
|
|---|---|---|---|
| FS2 |
3 |
63 |
66 |
| Surgicel |
13 |
56 |
69 |
|
16 |
119 |
135 |
| Test |
ChiSquare |
Prob>ChiSq |
|---|---|---|
| Likelihood Ratio |
7.084 |
0.0078 |
| Pearson |
6.598 |
0.0102 |
| Fisher's Exact Test |
Prob |
Alternative Hypothesis |
|---|---|---|
| Left |
0.0093 |
Prob(SUCDC=Yes) is greater for TREATN=FS2 than Surgicel |
| Right |
0.9984 |
Prob(SUCDC=Yes) is greater for TREATN=Surgicel than FS2 |
| 2-Tail |
0.0148 |
Prob(SUCDC=Yes) is different across TREATN |
(Ib) Contingency Analysis of SUCDC By TREATN (PP)
TREATN By SUCDC
| Count Total % Col % Row % |
No |
Yes |
|
|---|---|---|---|
| FS2 |
3 |
59 |
62 |
| Surgicel |
13 |
52 |
65 |
|
16 |
111 |
127 |
| Test |
ChiSquare |
Prob>ChiSq |
|---|---|---|
| Likelihood Ratio |
7.109 |
0.0077 |
| Pearson |
6.624 |
0.0101 |
| Fisher's Exact Test |
Prob |
Alternative Hypothesis |
|---|---|---|
| Left |
0.0092 |
Prob(SUCDC=Yes) is greater for TREATN=FS2 than Surgicel |
| Right |
0.9984 |
Prob(SUCDC=Yes) is greater for TREATN=Surgicel than FS2 |
| 2-Tail |
0.0144 |
Prob(SUCDC=Yes) is different across TREATN |
(II) Contingency Analysis of HAE4DC By TREATN
TREATN By HAE4DC
| Count Total % Col % Row % |
No |
Yes |
|
|---|---|---|---|
| FS2 |
16 |
50 |
66 |
| Surgicel |
32 |
37 |
69 |
|
48 |
87 |
135 |
| Test |
ChiSquare |
Prob>ChiSq |
|---|---|---|
| Likelihood Ratio |
7.320 |
0.0068 |
| Pearson |
7.213 |
0.0072 |
| Fisher's Exact Test |
Prob |
Alternative Hypothesis |
|---|---|---|
| Left |
0.0059 |
Prob(HAE4DC=Yes) is greater for TREATN=FS2 than Surgicel |
| Right |
0.9981 |
Prob(HAE4DC=Yes) is greater for TREATN=Surgicel than FS2 |
| 2-Tail |
0.0114 |
Prob(HAE4DC=Yes) is different across TREATN |
(III) Contingency Analysis of HAE7DC By TREATN
TREATN By HAE4DC
| Count Total % Col % Row % |
No |
Yes |
|
|---|---|---|---|
| FS2 |
6 |
60 |
66 |
| Surgicel |
16 |
53 |
69 |
|
22 |
113 |
135 |
| Test |
ChiSquare |
Prob>ChiSq |
|---|---|---|
| Likelihood Ratio |
5.084 |
0.0242 |
| Pearson |
4.915 |
0.0266 |
| Fisher's Exact Test |
Prob |
Alternative Hypothesis |
|---|---|---|
| Left |
0.0226 |
Prob(HAE7DC=Yes) is greater for TREATN=FS2 than Surgicel |
| Right |
0.9937 |
Prob(HAE7DC=Yes) is greater for TREATN=Surgicel than FS2 |
| 2-Tail |
0.0354 |
Prob(HAE7DC=Yes) is different across TREATN |
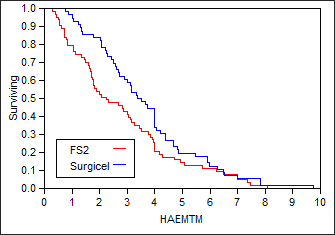
(IV) Product-Limit Survival Fit
Survival Plot
Time to event: HAEMTM Grouped by TREATN
Summary
| Group |
N Failed |
N Censored |
Mean |
Std Error |
|
|---|---|---|---|---|---|
| FS2 |
63 |
0 |
2.82857 |
0.25678 |
|
| Surgicel |
56 |
0 |
3.69821 |
0.26141 |
|
| Combined |
119 |
0 |
3.23782 |
0.18689 |
Quantiles
| Group |
Median Time |
Lower95% |
Upper95% |
25% Failures |
75% Failures |
|---|---|---|---|---|---|
| FS2 |
2.25 |
1.7167 |
3.1 |
1.1333 |
4 |
| Surgicel |
3.3667 |
2.7167 |
4 |
2.25 |
4.6667 |
| Combined |
3 |
2.4333 |
3.4333 |
1.6667 |
4.2 |
Tests Between Groups
| Test |
ChiSquare |
DF |
Prob>ChiSq |
|---|---|---|---|
| Log-Rank |
3.6733 |
1 |
0.0553 |
| Wilcoxon |
7.3567 |
1 |
0.0067 |