Taking New Steps to Meet the Challenges of Rare Diseases — FDA Marks the 11th Rare Disease Day
February 26, 2018
By: Scott Gottlieb, M.D.

FDA Commissioner Scott Gottlieb, MD
Today 30 million people in the United States – or one out of every 10 Americans – lives with at least one of more than 7,000 rare diseases. These conditions include rare cancers to inherited metabolic disease. And tragically, half of those affected by rare diseases are children.
This week, the U.S. observes the last day of February as Rare Disease Day![]() to raise awareness about rare diseases and their impact on patient’s lives. Unfortunately, finding treatments for these conditions does not become easier or less costly with the rarity of a disease.
to raise awareness about rare diseases and their impact on patient’s lives. Unfortunately, finding treatments for these conditions does not become easier or less costly with the rarity of a disease.
In many cases, developing a treatment for a rare disease can be especially hard and present unique challenges. Each success is the end of a long uphill climb. It requires the concerted efforts of many stakeholders, including scientists, product developers, regulators, policy makers, and of course, the energy and organization from patient advocacy groups.
For FDA, Rare Disease Day offers an opportunity to take measure of the progress we’ve made to help people affected by rare disease; and evaluate what more we can do to meet our commitment to advance the needs of patients with rare diseases and their families.
Thirty-five years ago there were few drugs and biologics for rare diseases and even fewer devices. Enacting the Orphan Drug Act in 1983 with its financial incentives and other inducements was an important start to enabling more investment and development of treatments targeted to rare diseases. Also important was legislation passed in 1990, creating a rare disease path for medical devices; known as the Humanitarian Device Exemption (HDE).
Since 1983, we’ve seen significant progress in treating rare diseases. FDA has approved more than 650 therapies for rare indications. This includes new molecular entities and biologics, as well as new rare disease indications for drugs approved for another indication. We’ve also seen progress in the development of devices for rare diseases. Since 1990, the FDA has approved 72 medical devices for an orphan indication under the agency’s HDE program.
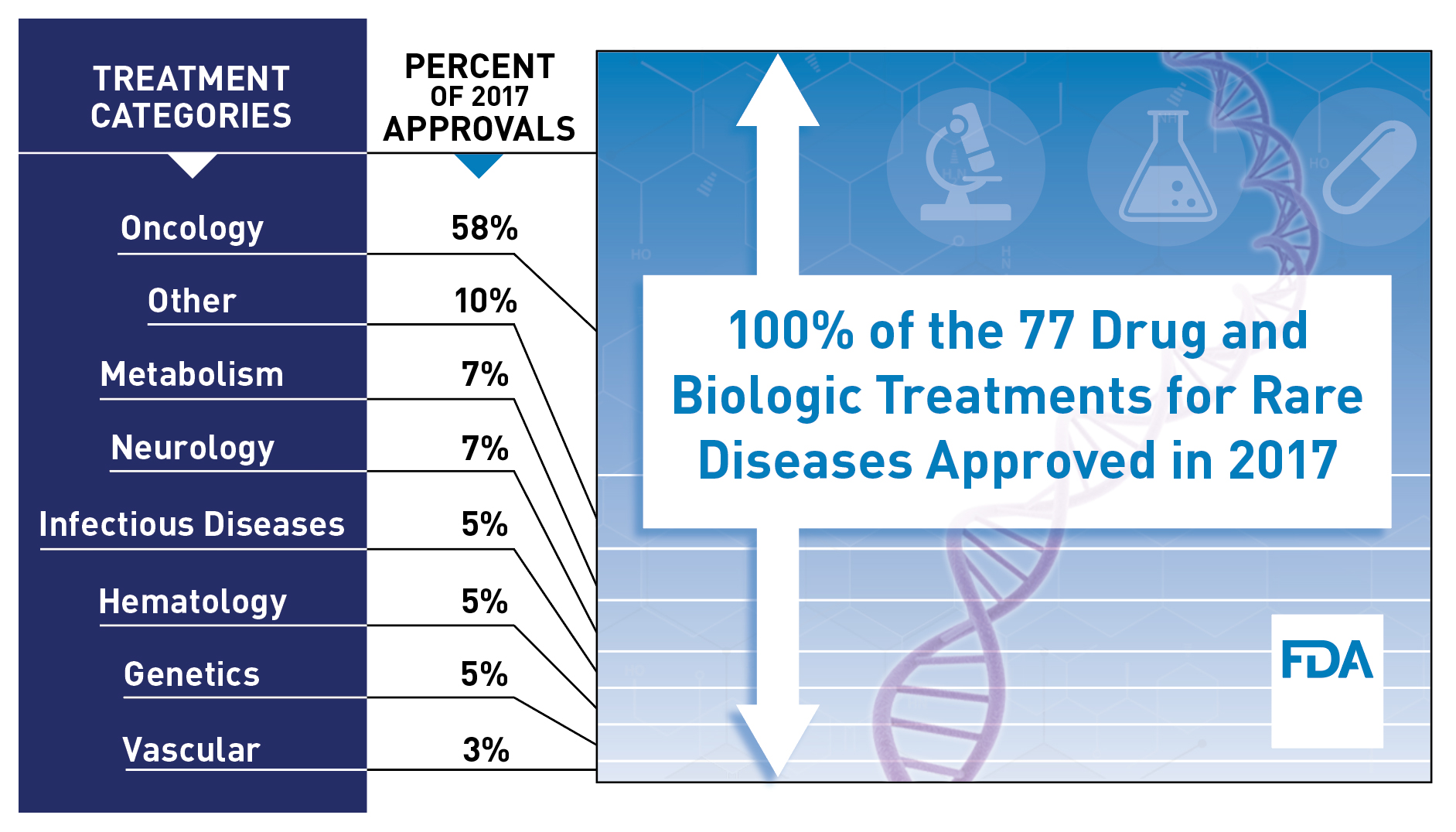
In recent years, the increasing emphasis on personalized medicine, including genetically targeted drug development, has enabled even more opportunities to develop treatments aimed at rare diseases. As a result, during the past five years, the number of requests to have a drug designated as serving an orphan population has steadily increased. In 2017, there were over 700 requests for designation. This was more than double the number of requests received in 2012. Last year we also saw 80 treatments approved by FDA for rare indications, the highest number ever.
FDA’s orphan drug program focuses its efforts on the full range of rare diseases, including relatively more rare or ultra-orphan diseases. In 2010, Miles Braun and other FDA researchers used data from 1983-2008 to show that there’s substantial effort with regard to the rarest diseases. The categories with the most orphan drug designations and the most approvals had very low prevalence levels. New analysis of more recent data shows this trend has been maintained. This experience suggests that the orphan drug program may continue to grow in importance as medicine becomes increasingly personalized, and better able to target the underlying molecular and genetic basis of even very uncommon disorders.
Despite these successes, we recognize that thousands of rare diseases still have no approved treatments. Indeed, FDA’s recent needs assessment survey, done in collaboration with the NIH’s National Center for Advancing Translational Sciences, identified a major public health need for innovative medical devices to care for children and adults with rare diseases.
FDA is committed to doing its part to facilitate continued progress toward more treatments and even potential cures for rare diseases. New scientific opportunities enabled by advances in cell and gene therapy hold out more opportunities to develop these potential cures. With efficient regulation, proper incentives for product development, and the continued support of patients, providers, and innovators; we’re more able to pursue these opportunities than ever before.
In June, I announced FDA’s Orphan Drug Designation Modernization Plan. Our aim was to create a more efficient, scientifically advanced, predictable, and modern approach to the approval of safe and effective treatments for rare diseases. Since then, we’ve eliminated the backlog of orphan drug designation requests. In addition, we’re fully implementing a 90-day timetable for processing new designation requests. We also established an FDA Orphan Products Council to further address scientific and regulatory challenges pertaining to orphan products.
Through our long-standing Orphan Products Grants Program we recently provided $17 million in funding to directly support 15 new clinical trials on products for rare diseases and to fund natural history studies for the first time. These four natural history studies, and an additional two studies funded through an NCATs partnership, could provide key information about how rare diseases develop and progress. This information can be vital for product development.
Of note, I also recently communicated our desire to expand upon these efforts to help foster investment and innovation in, and medical product development for, rare diseases by developing clinical trial networks to create an understanding of the natural history (such as individual patient experiences and progression of symptoms) and clinical outcomes of rare diseases. FDA’s 2019 budget includes a request for resources to make additional investments in rare disease natural history models. It’s clear more work can be done to advance these efforts.
Today I’m pleased to announce several new actions FDA is taking as part of our ongoing commitment to support and expedite the development of rare disease products. They include:
- A new pilot for more efficient orphan designation requests, including a new fillable form that will make the submission process easier for sponsors to complete designation requests; and make it more efficient for FDA to review. This also includes an on-line tutorial to guide sponsors through the submission process. We’ve also developed a new inter-center consult process to streamline and standardize our communications process.
- We are entering into a new Memorandum of Understanding with the National Organization for Rare Disorders to conduct outreach with our new Patient Affairs Staff on ways to enhance the incorporation of patient experience into regulatory discussions. As part of this process, we’re planning a joint series of pilot listening sessions on rare diseases. We recognize that early and iterative engagement can improve clinical and regulatory understanding of diseases and conditions; provide a common understanding of the most urgent patient needs; and inform drug development programs.
- We’re planning a public meeting that will help us prepare for the changing landscape of orphan drug development posed by the growth in targeted therapies and molecularly defined diseases. At an upcoming meeting, we’ll seek input on complex scientific and regulatory issues related to cancer drugs and biologics that target a tumor’s specific genetic features rather than its location in the body (i.e., tissue agnostic approvals). We’ll also consider the appropriate application of orphan incentives to this new paradigm of drug development, and how we apply designations to these indications.
To provide the public with a more complete discussion of the scope of FDA’s rare disease activities, we’ve also created a new, enhanced web page that features videos from our three center directors plus other materials. I invite you to take a look.
Over the course of 2018 we’ll continue our efforts to increase the consistency and efficiency of our reviews of rare disease products. We remain committed to supporting rare disease research on diagnostics, therapies, and potential cures. We’ll also continue to evaluate how to best support investment in rare diseases; and to encourage the development of drugs that target rare, unmet patient needs. A lot of devastating and rare conditions still lack approved therapy. During this Rare Disease Week, it’s gratifying to review the steps we’ve taken, and to commit to more progress in the future, and making sure that our framework supports the needs of patients.
Scott Gottlieb, M.D., is Commissioner of the U.S. Food and Drug Administration