Sampling Program Provides Scientific Foundation to Better Protect Consumers from Foodborne Pathogens
By: Susan Mayne, Ph.D., Director, Center for Food Safety and Applied Nutrition (CFSAN), and William Correll, Director, Office of Compliance, CFSAN
The FDA is charged with the critical responsibility of protecting major portions of the nation’s food supply from hazards that could endanger public health. One of the ways in which the FDA works to keep the food supply safe is to conduct surveillance sampling of produce and processed foods. Collecting data on the prevalence of pathogen contamination helps the FDA focus its response and regulatory actions and helps prevent contaminated products from reaching consumers.
The agency has been conducting surveillance sampling of the food supply for decades but enhanced the pathogen surveillance program in 2014 to be more proactive and preventive by selectively targeting certain food products for testing. This is different from the “for-cause” sampling that is conducted in response to a known contamination of food, such as during an outbreak of foodborne illness. In the surveillance approach, which complements the FDA’s smaller-scale assignments, the agency selects certain commodities for a larger sampling exercise of approximately 1,600 samples of each over 12 to 24 months. Obtaining a dataset of that size is key because it provides the agency with statistical horsepower. With 1,600 samples, if there is contamination in a targeted commodity, the agency is likely to detect it, and the precision of our estimate of the prevalence of contamination is much greater. The FDA can also look for subgroups of a commodity in which there may be a higher prevalence of contamination. If the agency encounters signals that positive samples come from a specific geographic region, it can initiate separate, targeted sampling activities. This flexibility allows the agency to be responsive to what the data indicate and can help clarify the extent to which contamination may be a concern for the sampled food while also allowing us to branch off onto other avenues when appropriate.
After completing each assignment and analyzing our findings, we decide if additional studies or mitigation efforts are needed. When sampling findings, combined with other data, indicate a relatively low risk, the FDA may focus fewer future resources on that commodity. When sampling findings have helped the FDA better understand potential risks associated with that commodity, the agency may develop interventions to help address the risks. These could include education and outreach to industry or consumers, updated policies, or targeted compliance activities. And finally, if key knowledge gaps remain after analyzing our findings, we may encourage or initiate additional activities to fill these gaps, including additional sampling assignments or research studies.
We get input early on from industry, regulatory partners
Staff in the FDA’s Office of Compliance and their colleagues in the agency’s Division of Risk and Decision Analysis begin by developing a list of food-pathogen combinations that are good candidates for sampling, based largely on the history of detection in foods, recalls, and outbreak history. In prioritizing sampling efforts, other factors are considered, including the severity of illness, the characteristics of the food and its manufacturing process, and whether large-scale sampling is an appropriate tool for filling current knowledge gaps.
Next, the FDA collaborates with industry associations and regulatory partners — before conducting any sampling — to get feedback on our list and approach to sample collection. We ask if they have or know of existing data on the products we are considering sampling. This outreach, coupled with our own research, holds open the possibility that we may not need to sample a given food, if in fact we can obtain data from other rigorous studies.
Assignments are designed in a way that minimizes impact on industry operations. Time is of the essence in obtaining test results when it comes to produce and other perishable foods, as no one wants to waste food, and the FDA is sensitive to the economic concerns of industry. As part of our outreach, we work with industry to make our sampling, analytical testing, and reporting back of findings as efficient as possible, and we routinely incorporate the suggestions we receive into our planning.
After considering all feedback, the agency selects foods for the following year’s program. Since the program’s initiation in 2014, we have conducted work on two to three commodities per year.
Sample collection and testing
To ensure the sampling is as representative as possible, we collect samples in approximate proportion to their U.S. market share based on origin (i.e., domestic vs. import). For example, if 60 percent of a commodity eaten by U.S. consumers is imported, then about 60 percent of the samples of that commodity should be from imported products. This approach helps approximate the products and relative risks that consumers are likely to encounter.
We don’t share the specific locations where we’ll be sampling, or which business’ products might be collected for testing. It is important to get an unbiased snapshot of the frequency of contamination in the commodity, and we achieve that only with unannounced collection visits.
Once the assignment is underway, it typically takes a few days to obtain initial test results from each set of samples, depending on the microbial hazards targeted. We share test results, positive or negative, with firms as soon as they become available. Some firms opt to hold distribution of their product until we notify them of test results. If potentially harmful contaminants are found in a product that has been distributed or is on the market, the FDA will take appropriate regulatory and enforcement action, including recall.
The FDA routinely updates the public on largescale assignments that are underway. The results to date on the agency’s current sampling of fresh herbs (basil, cilantro and parsley), and processed avocado and guacamole, are posted at FDA.gov.
Benefits for everyone
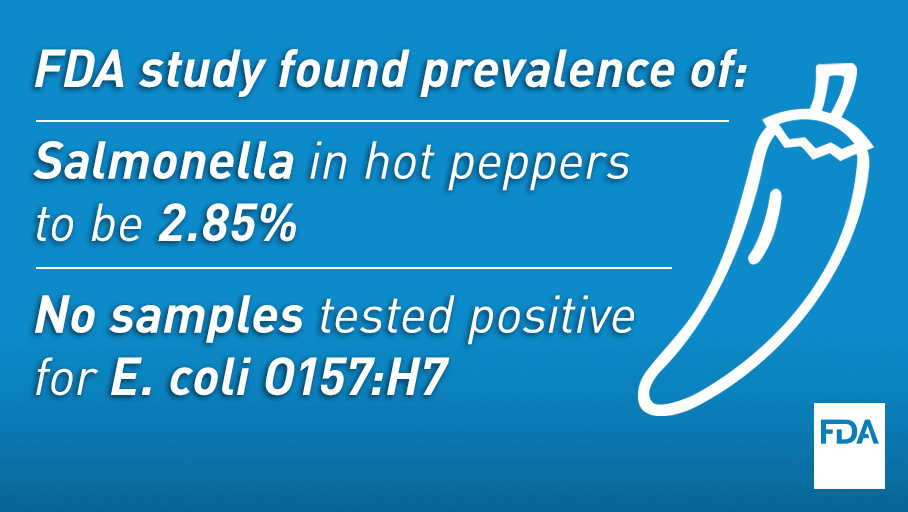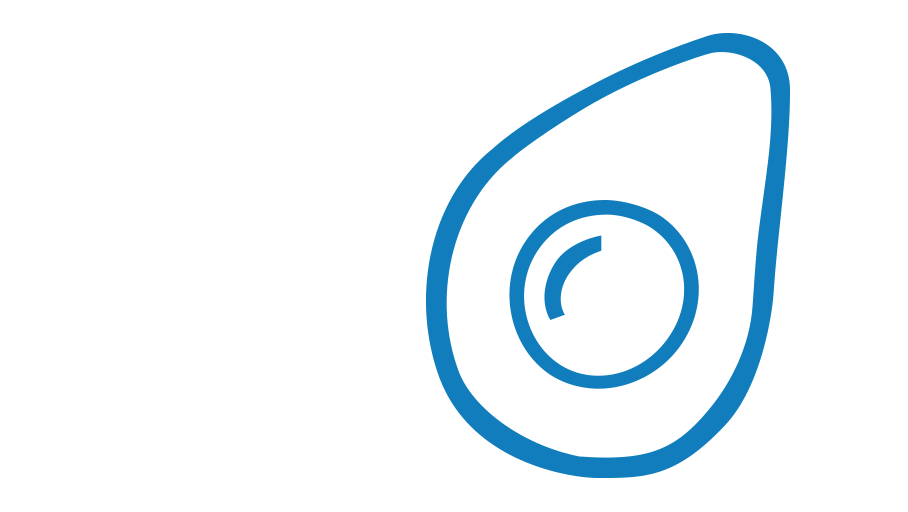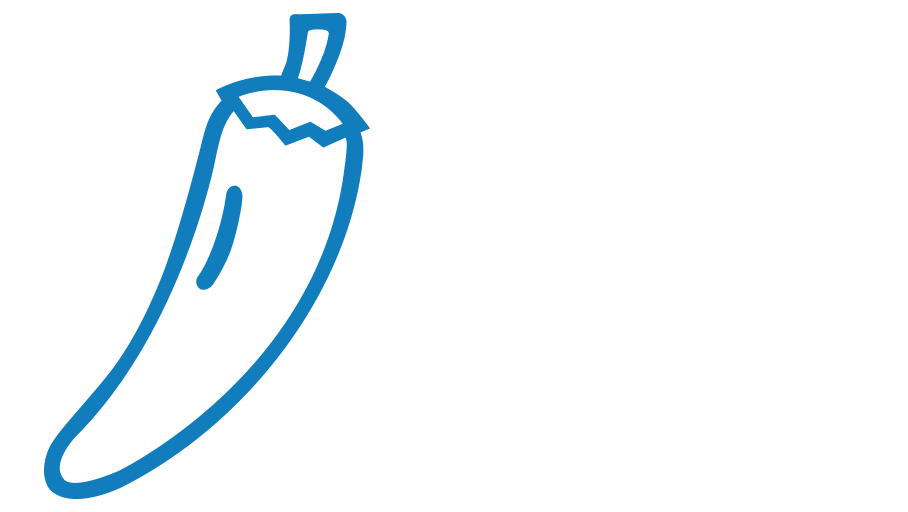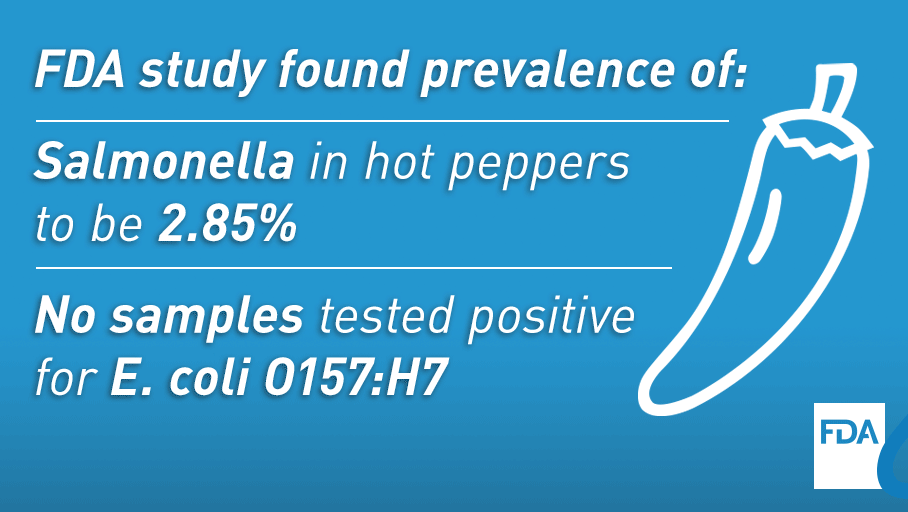
This sampling has demonstrated tangible benefits for public health. For example, during our FY2014 to FY2016 sprouts assignment, our investigation of positive samples resulted in six voluntary recalls, among other actions. Most significantly, the assignment helped stop an outbreak of listeriosis while it was still small in case count. In 2016, while conducting a hot peppers assignment, we helped identify hot peppers as the likely cause of a multistate outbreak of salmonellosis that involved 32 people.
Equally as important, these assignments are creating greater industry awareness and helping elicit needed changes on the part of firms. While industry is responsible for ensuring that the food it produces is safe, these largescale assignments provide the big picture with respect to contamination frequencies in a given commodity. We can then use that information as a basis for working with industry to identify ways to further minimize hazards.
Most recently, the agency released two reports on commodities sampled under our surveillance sampling program, one on avocados and the other on hot peppers.
The sampling program process is already improving outcomes and facilitating collaboration between the FDA and industry, to the benefit of consumers. During the avocado assignment, we determined that the prevalence of Listeria monocytogenes contamination on the skin of the fruit was higher than expected. That pathogen can be transferred to the avocado pulp during preparation for processed foods, and so we decided to conduct a follow-up assignment to examine processed avocado and guacamole. Early in the follow-up sampling assignment in November 2017, we identified a contaminated guacamole product that the processor then immediately recalled while also temporarily ceasing production to investigate and take preventive measures for future production. Our focused sampling and sharing of our interim findings increased awareness of microbial risks associated with the fruit on the part of industry and thus served to inform hazard analyses conducted to implement preventive controls. Importantly, in carrying out our two avocado assignments, we learned that several largescale processors are using in-package, high-pressure pasteurization, a ‘kill step,’ to eliminate pathogens. We will continue work with industry and other food safety experts on best practices that may be used to reduce contamination of avocado skin with Listeria monocytogenes. Using science and technology to address foreseeable food safety risks helps protect consumers from contaminated foods.
The FDA’s enhanced sampling program has strengthened the agency’s ability to detect contamination in selected foods, and whole genome sequencing, coupled with epidemiological information, has improved attribution of illnesses to the microbial hazards that may have caused them. We recognize the importance that traceability has on better pinpointing the sources of contamination so that we can quickly intervene to stop outbreaks while case counts are small in number or, wherever possible, to forestall illnesses altogether.
The FDA will continue building this sampling program, working with public and private partners to gain data-driven knowledge of potential hazards in the food supply. Armed with that knowledge, the agency’s ability to mitigate risks will evolve and strengthen, giving consumers the confidence they deserve to have in the safety of the food supply.