GUIDE TO TRACEBACK OF FRESH FRUITS AND VEGETABLES IMPLICATED IN EPIDEMIOLOGICAL INVESTIGATIONS April 2001
Last updated June 2006
ATTACHMENTS
Attachment 1 – PAC Codes
Attachment 2 – Traceback Data Analysis Example
Attachment 3 – Multi-State Flow Diagram Example
Attachment 4 – Creating Electronic Documents
Attachment 5 – Interview Questions
INTRODUCTION
A product investigation begins when a food is suspected or implicated in a foodborne outbreak. Product investigations can include facility inspections, food preparation reviews, sample collections, and environmental, traceback, and source/farm investigations. A food can be implicated or associated with foodborne outbreak through one or more of the following methods: epidemiological or statistical, laboratory, and/or a thorough food preparation review.
A traceback investigation is the method used to determine and document the distribution and production chain, and the source(s) of a product that has been implicated in a foodborne illness investigation. A traceback investigation involves good interviewing techniques, a complete record review, and timely reporting to meet its intended purpose. A subsequent source investigation may be conducted to determine possible routes or points of contamination by inspecting common distribution sites, processors, and/or growers identified in the traceback investigation.
A traceback investigation may be conducted for several reasons: 1) to identify the source and distribution of the implicated food and remove the contaminated product from the marketplace, 2) to distinguish between two or more implicated food products, and 3) to determine potential routes and/or sources of contamination in order to prevent future illnesses.
CDC or state/local health or regulatory agencies may conduct limited tracebacks and/or traceforward investigations in order to strengthen an epidemiological association by comparing the distribution of illnesses and the distribution of the product. This is often referred to as an “epi” traceback.
Fresh fruits and vegetables are extremely difficult to traceback because they are perishable commodities and lot numbers and grower identifications are not routinely used or recorded on shipping records. If the distributor cannot identify specific shipments and their source, the tools and interviewing techniques outlined in these instructions will help identify possible shipment(s), suppliers, and the source(s) involved in the outbreak. The procedures outlined in this guide are not to be used for tracebacks involving eggs.
Investigators working on multi-state foodborne outbreak investigations will want to refer to the National Food Safety System manual, Multi-State Foodborne Outbreak Investigations: Guidelines for Improving Coordination and Communications at http://www.fda.gov/ora/fed_state.
This traceback guide is presented in three main sections: 1) Traceback Procedures, 2) Methods and Analysis and 3) Farm/Source investigations. It also includes examples of the analysis process and instructions for creating electronic traceback documents.
TRACEBACK PROCEDURES
INITIATING A TRACEBACK INVESTIGATION
Initiation of a traceback investigation usually begins when 1) epidemiological evidence implicates a food product and 2) hazard analysis shows that other contributing factors were not to blame (e.g., cross-contamination, ill food workers, other on-site sources of infectious agent). Other factors that will be considered prior to initiating a traceback investigation include disease severity, the risk of ongoing exposure, the availability of shipping records, reliable exposure data, the size and scope of the outbreak(s), and the availability of resources to conduct the investigations.
FDA Emergency Operations Center (EOC) should be notified when a District Office is aware of a foodborne outbreak or when a state/local agency initiates a traceback investigation of a product that has the potential to fall under FDA jurisdiction. The District Offices should refer all requests for FDA to participate in or to conduct a traceback investigation to EOC (301-443-1240). EOC will provide early alerts to the District Offices and CFSAN when a traceback investigation has been requested and/or may be assigned in the near future.
EOC may ask a District to obtain the following information from the state/local agencies: a written epidemiologic summary, a hazard analysis or environmental and inspection reports (including a food preparation review), laboratory results, and copies of any invoices and distribution information that have been collected. If CDC conducted or coordinated the epidemiological investigation, EOC will request the information from CDC.
The available data will be reviewed by EOC and CFSAN. At the request of FDA, epidemiologists from other agencies, such as CDC, may also be asked to review the epidemiological data. EOC will consult with CFSAN and determine if a traceback is to be initiated. EOC will generate a traceback assignment in FACTS. All inter-district traceback work requests will be assigned in FACTS by EOC.
TRACEBACK COORDINATION
When a traceback investigation has been assigned to you, your District should contact EOC. The EOC coordinator will then initiate a conference call with you to discuss the assignment. Your District may wish to include your supervisor or other designated person(s) on this initial call. You will have frequent communication with the EOC coordinator throughout the investigation. Upon completion of each traceback step, you should contact the EOC coordinator to discuss the data analysis and the next stage of the traceback. The District may choose to have other individuals participate in these ongoing discussions, for training or other reasons. After each firm visit, a copy of the records you collected should be faxed to EOC. If there are a large number of records, they can be sent by overnight mail.
There are several reasons why this communication and coordination with EOC is a critical component of traceback investigations. The primary reason is that most traceback investigations are in response to multi-state foodborne outbreaks and therefore tracebacks are usually occurring simultaneously in multiple Districts. The EOC coordinator will be the main communication point, receiving and analyzing information from multiple districts that will help to guide the investigations. This central coordination will allow earlier notification to the District Offices, provide the District management with greater flexibility in assigning traceback work, provide training opportunities for investigators, eliminate repetitive or unnecessary work, and improve FDA’s overall response time.
PRODUCT SAMPLES
If leftover food from the implicated meal(s) or product from an implicated shipment is available, it should be collected. Contact EOC for instructions on where to send the samples and what analysis to request. Unless otherwise directed, product from later shipments should not be collected.
USE OF FDA REPORTS AND FORMS
FDA 463A – Affidavits are not required or encouraged to be used in a traceback investigation. All pertinent information and discrepancies that are noted during the investigation should be explained in the memo. The following are examples when you may chose to use an affidavit during a traceback investigation:
- to provide an explanation of inaccurate shipping records or written mistakes on records that cannot be verified or documented using other records but can be explained by an employee.
- to describe the personal knowledge or belief of an employee regarding the shipping and/or handling of the implicated product which is not supported by other documents or other interviews and would have a direct bearing on the analysis of the records.
FDA 482, Notice of Inspection - an FDA 482 should be issued to each firm that is visited in the course of the traceback investigation. Memo - if you conduct a food preparation review at the point of service, this information can be reported in a memo. FDA 483 List of Observations, Establishment Inspection Report, and FMD 145 Post-inspection Letter - if you conduct a domestic establishment inspection as a result of a traceback investigation, the procedures regarding these inspections and forms are outlined in the IOM and should followed.
TRACEBACK REPORTS
You will be responsible for generating a report for the portion of the traceback investigation that was assigned to you. The traceback report should consist of a FACTS cover sheet with your supervisor’s endorsement and the FOI statement, a timeline and the flow diagram(s). The following items should also be included for each firm you visited:
- FDA 482
- Invoices, inventory records, shipping/receiving records (including records and information obtained from other agencies).
- A memo summarizing the information gathered from the observations and interviews at the firm, including explanations of the data analysis (i.e., how receipt dates were determined).
- Affidavits (if used).
In addition to the flow diagram that you received from EOC at the completion of their assignment, EOC will also send a final flow diagram to you when the entire investigation is complete. If the traceback was part of a multi-state outbreak, and more than one traceback was initiated, there may be a third flow diagram that will be sent to you that shows the results of all of the tracebacks. Your traceback report should be maintained in your District Office and a copy should be sent to EOC. The copy that is sent to EOC does not need to include a copy of the records, as these have already been sent to EOC.
REQUESTS FOR INFORMATION
FOI Requests
You should include the following statement in your final traceback report: “All FOI requests or other inquiries for release of foodborne outbreak and traceback investigations should be referred to EOC (HFC-160). ” It is recommended that this statement be in bold lettering at the bottom of the cover page and may be included as part of your supervisor’s endorsement. Some of the information collected in the course of these investigations is non-public, commercial confidential information.
Release of Information to FDA Commissioned Officers
Prior to releasing any foodborne outbreak and traceback documents to a state or local official who is an FDA Commissioned Officer, you must contact EOC. EOC will work with DFSR to verify the status of the commission and the 20.88 Certification Commitment form to determine what information can be released.
You, the investigator, are responsible for completing the following tasks:
TRACEBACK RESPONSIBILITIES
- If requested, obtain the following information from the state/local agencies (if available) and provide a copy to EOC:
- Epidemiological data
- Exposure dates
- Exposure places
- Environmental inspection
- Food worker health
- Cross-contamination issues
- Preliminary traceback and distribution information
- Implicated product name and any available packaging, labeling
- Epidemiological data
- Obtain the following from EOC:
- Coordinator name and contact number(s)
- Dates for record collection
- FACTS Assignment Number
- Traceback Event Code
- Timeline
- Flow Diagram
- Review the background information with EOC prior to visiting a firm.
- Conduct an investigation and record collection at each implicated firm, in the order it is assigned.
- Analyze the data. Fax (or overnight) a copy of the records to EOC after each firm is visited. Discuss analysis and next steps with EOC Coordinator before proceeding to the next implicated firm.
- Repeat Steps 4 and 5 until either a potential source (manufacturer, grower, producer, importer) is identified or an implicated firm is located out of your area of responsibility.
- Summarize each firm investigation in a memo.
- Send EOC a copy of the traceback report.
- Coordinate with state and/or local investigators. A courtesy call should be made to both the state health and state agriculture representative when a traceback is initiated in the state. DFSR and EOC can assist you with this notification.
- Lead or participate in domestic and/or foreign source investigations, if requested.
The EOC Coordinator is responsible for completing the following tasks:
- Obtain a written summary of all available epidemiological, environmental and laboratory data from CDC (if available) or the District Office(s).
- Generate FACTS assignments and Traceback Event Codes.
- Obtain the shelf life of the product and any other product information.
- Set the parameters for the dates of the records collection.
- Initiate a conference call with investigator to discuss and share the background information on the outbreaks, the product, and the assignment.
- Maintain regular contact with the investigator.
- Review traceback records and data analysis.
- Conduct or assist with data analysis, as needed.
- Provide training to investigators, as needed.
- Update and distribute the timeline and flow diagram to the investigators throughout investigation.
- IssueInter-Districttraceback assignments in FACTS.
- Compile a final report for EOC that includes each investigator’s report and the final timelines and flow diagrams.
- Work with District management to identify investigators to lead and participate in domestic and foreign source investigations. Participate in domestic and foreign source investigations, as needed.
- Communicate findings to the District Investigators and CFSAN. Provide a final flow diagram to all of the Districts that participated and CFSAN when the traceback(s) are complete. Using appropriate confidentiality agreements, release findings to other agencies (i.e., CDC, USDA, State agencies) when requested and approved by EOC management.
- Complete FOI requests using guidelines pertaining to release of Commercial Confidential Information. EOC Management should review the documents prior to release of information.
CFSAN’s responsibilities are described in the “CFSAN Emergency Response Procedures”, July 25, 2000.
METHODS AND ANALYSIS
If an outbreak involves multiple points-of-service, each traceback will be conducted and analyzed individually and then analyzed as part of the overall traceback investigation. Appendix B provides an example of the analysis process for a point-of-service outbreak. Appendix C illustrates how multiple points-of-service tracebacks are analyzed.
POINT-OF-SERVICE (POS) INVESTIGATION
The goal of the point-of-service (POS) investigation is to determine and understand the firm’s ordering, receiving, stock rotation, inventory, and preparation procedures and to obtain the appropriate records and documentation. By understanding how the product is received, recorded, rotated and prepared, you will be able to determine which shipments were available and most likely consumed at the implicated meal.
It is also important to rule out on-site contamination as a contributing factor especially when it is a single point-of-service outbreak. If the local or state agencies have not conducted a food preparation review or if it is not sufficient for FDA’s needs, you are encouraged to contact your Regional Retail Food Specialist, who may be able to assist with that part of the investigation. Cross-contamination or on-site contamination is less of a concern in multi-state or multiple points-of-service outbreaks. In some situations, a food preparation review may not be necessary.
In addition to the record collection and review, interviews and observations are key parts of a traceback investigation. It is not possible to do a thorough traceback investigation over the telephone. You will need to conduct the interviews, make observations, and collect records in person.
POS Interviews
If the POS is someone’s home or a non-traditional place of business, the exposure data and other product information will be collected and verified by the state health agency and reviewed by FDA prior to the initiation of the traceback. In these instances, the place the consumer(s) purchased the food will be considered the point of service (e.g., grocery store, caterer).
You should conduct interviews with more than one employee at multiple levels of the organization (e.g., chef, kitchen manager, line cook) to determine the following information:
- Product Identifying Information- Include pertinent label information (collect copy if available), container type, size, color, grade, and lot codes, production or pull dates if available, and product origin. The firm may be using a different brand than what it was using at the time of the outbreak. Verify label, product information with invoices and shipping receipts for the time period in question. Only collect product label information for the product that was used during the outbreak exposure time period.
- Shipping and Receiving Practices - Determine the receiving dates and times for each shipment in the requested time period. Indicate how the dates on the shipping records reflect the date the product was received. Determine how supplier deliveries are documented or recorded. Determine the firm’s suppliers during this time period, including any cash transactions. Determine or estimate the transportation time from the supplier to the point-of-service.
- Handling and Storage Practices - Interview employees regarding receipt and preparation of the implicated product for the implicated meal, if this information hasn't already been provided by CDC, state, or local authorities.
- Stock rotation practices - Review the standard operating procedure at the firm. Determine how the product is unloaded and added to existing inventory. Determine if a first-in-first-out (FIFO) rotation policy is standard operating procedure and how closely it is adhered to.
- Daily (or otherwise) stock inventory - If an inventory record is available for this time period, understand how it is used, including its strengths and weaknesses, and determine what time of day the inventory is performed. Understand what each inventory number represents. Determine how partial cases or containers are accounted for, and how and if carry over is recorded.
- Ordering practices – Determine how and when product is ordered. Determine average daily use.
POS Observations
You should observe and verify that the procedures described by employees are reflected in their actual work. Whenever possible, have the employees who regularly perform each of the tasks demonstrate their procedure. You should be able to describe the flow of the product through the firm. Walk through the receiving and shipping process and follow a shipment of product physically through the firm and compare it with the records (written and computerized). This information will be critical for the analysis.
POS Record Collection
You should obtain a copy of all invoices, shipping and receiving records, bills-of-lading, inventory records and any other records regarding the implicated product that are available over the time period requested.
You will want to review the records on site. There will often be many dates and handwritten notes on the records. You should determine the estimated date of receipt. The person who is responsible for receiving the product should be able to assist in this. The shipping date, the invoice date, and/or the order date may be the same as the receipt date or they may be used to determine the receipt date. Do not make assumptions regarding the dates on the records. Be sure to ask for an explanation of all dates on the records.
Interview the employees and determine the date and time shipments are received. Determine the transit time from the distributor shipping the product to the POS. You will need to clearly document in the memo how the receipt date was determined and how it relates to the dates on the records. You should be able to read and understand all items on the records. If the quantity printed on the record is difficult to read, it may be calculated using the unit price and total price. This is the only time that the price would be of interest. Some suppliers may be reluctant to disclose pricing information. If the quantity is legible, there is no reason why FDA needs the pricing information. If the supplier prefers to redact the pricing information before releasing the records that is acceptable and will not affect FDA’s investigation. You may encounter resistance when requesting records. You should take the time to explain the reason for this investigation, including a summary of the outbreak, the number of illnesses, and the importance of identifying the source of the product. Be prepared to volunteer to go through the firm’s files to locate the records, assist clerical help in doing so, make the copies (on-site or off-site), and go to other locations where the records may be located.
If you are unable to obtain the records immediately, you should be persistent in obtaining them as quickly as possible. If you are not able to get the records in a timely manner (1-2 days), the EOC Coordinator should be notified immediately. Many states have laws that require these shipping records to be provided to the State health and regulatory authorities upon request. If the firm will not provide the requested records, you may ask for assistance from these agencies.
Analysis of Point-of Service Data
You will need to analyze the first level of data before continuing the investigation at another firm. If you are being trained or would like assistance with the analysis, you should contact your EOC Coordinator. EOC will provide an electronic timeline for you to use in your analysis. The data analysis will be described in this section and Appendix B provides an example of the analysis process. You will need to follow these steps in order to analyze the data.
Step 1. Use the timeline provided by EOC. The timeline should be labeled with the following:
- Title, Date, Event Code, Product
- Dates of Record Collection
- POS Firm name
Step 2. Fill in Receipt Dates.
- Add the names of the suppliers under the POS, one per line.
- If an inventory list is available, place the quantity on the POS line corresponding to the appropriate date. You will need to note in the memo how and when inventory is taken.
- Place the quantity of each shipment in the cell corresponding with the date it was receivedat the POS from the corresponding supplier.
Step 3. Review the pertinent information collected during the interviews and observations.
- How the receipt date is determined
- How inventory is handled
- Delivery receiving times
- Serving and preparation times
- Stock rotation practices
Step 4. Implicate shipments and suppliers. Using the information from the interviews and observations, determine which shipments received at the point of service were available and could have been used in preparing the implicated food item. This will tell you which firms supplied those shipments.
Step 5. Contact EOC.
- Fax (301-827-3333) or overnight (5600 Fishers LaneRm 12A55 Rockville, MD20857) a copy of the invoices to EOC.
- Discuss the analysis with the EOC Coordinator. You will need to share the information you have added to the timeline. You may fax this or communicate the information by phone.
- If the implicated firm is in your district, you will continue the traceback at the implicated supplier (if it is assigned to you). EOC will add the firm to the FACTS assignment.
- EOC will provide an updated electronic copy of the timeline and flow diagram to you.
Do not write the traceback report until you have completed all of the implicated firms assigned to you. You may only have the point of service firm located in your district. If that is the case, then the report should be written at this time. However, if the implicated supplier(s) is/are in your District, the continuation of the traceback investigation should not be put on hold while a report for the point-of-service firm is written. When the traceback investigation is completed, the final flow diagram(s) will be sent by EOC to CFSAN and to each investigator who participated in the traceback.
DISTRIBUTOR INVESTIGATIONS
The goal of the investigations at the implicated suppliers or distributors is similar to the goal at the point of service. You will not need to determine preparation procedures and serving times. You will need to verify all previously collected shipping information from the distributor to the point-of service and also collect new information on all incoming receipts of product to this distributor from other distributors, processors, importers or growers. Interviews, observations and record collections should be done in person. You may or may not have conducted the point-of-service investigation. It is important for you to review the point-of-service analysis and shipping receipts prior to beginning the investigation at a distributor.
There are several types of distributors and other firms that may be encountered during the traceback investigation: distributors that supply to the point of service, distributors that supply product to other distributors, and brokers who may never physically handle the product, only the paperwork. There may be transportation firms and cold storage facilities whose paperwork may be available at a distributor. You will not usually be asked to visit the transporter or storage facility, but you should collect any of this related paperwork during your investigations at the distributor.
You will be less likely to find inventory records that accurately reflect the physical inventory at the distributor level. Many distributors will use a computerized first-in-first-out inventory system. It will not usually match exactly what is occurring on the warehouse floor. You will need to take the time to understand how this system works and if it can be used for analysis. Again, observation and interviews will be critical. You should follow the instructions that were provided for the POS investigation. Interview different employees, observe their practices, and determine if the written procedures reflect what is actually being done.
If the firm is able to link incoming and outgoing shipments, first determine the accuracy of the system through observations and interviews and then by “walking through the process” before using this information in the analysis.
Distributor Interviews
You should conduct interviews with more than one employee at multiple levels of the organization (e.g., floor manager, loading dock personnel, shipping clerks, drivers, etc.) to determine the following information:
- Product Identifying Information – Verify that all information that is collected pertains to the implicated product. Check brand names, product descriptions, etc. The firm may be using a different brand or multiple brands than was shipped during the time frame under investigation. Verify label and product information with invoices and shipping receipts for the time period in question. Only collect records for the implicated product using label information provided by the point-of-service. The record collection dates for the distributor will cover the same time period that was assigned at the point-of-service.
- Shipping and Receiving Practices - Determine the receiving dates and times for each shipment in the requested time period. Indicate how the dates on the shipping records reflect the date the product was received. Determine how supplier deliveries are documented or recorded. Determine the firm’s suppliers during this time period, including any cash transactions. Determine or estimate the transportation time from the supplier to the point-of-service.
- Stock rotation practices - Understand the standard operating procedure at the firm. Determine how the product is unloaded and added to existing inventory. Determine if a first-in-first-out (FIFO) rotation policy is standard operating procedure and how closely it is followed.
- Daily (or otherwise) stock inventory - If an inventory record is available for this time period, understand how it is used, including its strengths and weaknesses, and determine what time of day the inventory is performed. Understand what each inventory number represents. Determine how partial cases or containers are accounted for, and how and if carry over is recorded.
- Ordering practices – Determine how and when product is ordered. Determine average daily use.
- Product Handling and Storage Practices - Interview multiple organizational levels regarding receiving, repackaging, and/or handling of the implicated product prior to delivery to the point-of-service. Report on relevant unloading, storage, loading or other pertinent conditions. If the implicated product is repackaged, processed or handled in a way that could expose it to possible contamination, you should make a note of this and include it in the report memo. An inspection may be needed at a later date if this firm is determined to be a common point of distribution in the overall traceback. This cannot be determined until the traceback is fully completed. Unless otherwise instructed to do so, do not complete a GMP inspection at this time. In most cases, this is unnecessary and would cause a significant delay in the traceback investigation. If GMP violations are observed during the traceback investigation, notify District management immediately and then EOC.
Distributor Observations
You should observe and verify that the procedures described by employees are reflected in their actual work. Whenever possible, have the employees who perform each of the tasks demonstrate their procedure. You should be able to describe the flow of the product through the firm. Walk through the process and follow a shipment of product physically through the firm and compare it with the written (and computerized) records. This information will be critical for the analysis.
Distributor Record Collection
You will need to verify that the shipment information collected at the point-of-service from this distributor is both accurate and complete. This will involve looking at all outgoing shipments from this firm to the point-of-service firm. You do not need to recopy these records. If a previously unidentified shipment is found, you should copy that record. You do not need to collect information on outgoing shipments from this firm to firms other than the POS, unless directed to do so. You will need to collect and review all receiving records for all incoming shipments of the implicated product over the same time period. Refer to the point-of-service investigation for additional guidance on record collection and review.
Analysis of Distributor Data
You will need to analyze this data before proceeding to the next level of distribution. If you are receiving training or would like assistance, you should contact the EOC Coordinator. The analysis will be described in this section. Appendix B includes an example for you to follow.
Step 1. Using the updated timeline provided by EOC, add the following information:
- Add the names of the suppliers under the distributor, one per line.
- If an inventory list is available, place the quantity on the distributor line corresponding to the appropriate date. You will need to note in the memo how and when inventory is taken.
Step 2. Fill in Receipt Dates. Place the quantity of each shipment in the cell corresponding with the supplier and the date it was received at the firm.
Step 3. Review the pertinent information from interviews and observations
- How the receipt date is determined
- How inventory is handled
- Stock rotation practices
- Time and dates product is received and shipped
Step 4. Implicate shipments and suppliers.
- Using the information from the interviews and observations, determine which shipments received at this distributor were available and could have been used to fill the implicated shipments.
Step 5. Contact EOC.
- Fax (or overnight) EOC a copy of the invoices.
- Discuss the analysis with the EOC Coordinator. You will need to share the information you have added to the timeline. You may fax this or communicate the information by phone.
- If the implicated firm is in your district, you will continue the traceback at the implicated supplier (if it is assigned to you).
- EOC will update and provide an electronic copy of the timeline and flow diagram to you.
Do not write the traceback report until all of the investigations of implicated firms assigned to you are completed. You may have more than one distribution firm located in your District. If additional implicated suppliers are in your District and assigned to you, the continuation of the traceback investigation should not be put on hold while a report is written. Once you have completed all the assigned firm visits, the report should be written and a copy sent to EOC. When the traceback investigation is completed, final flow diagram will be sent by EOC to CFSAN and to each investigator that participated in the traceback.
IMPORTER, PROCESSOR, AND GROWER INVESTIGATIONS
If an implicated firm is an importer, you should conduct an investigation in the same manner as a distributor investigation. However, you will need to work with the importer to determine the country of origin and the source of the product. There will be additional records that will usually be available at the importer level such as airway bills and customs entry documents. Collect all available records related to the implicated shipments.
If a grower or processor is implicated, you should notify EOC. A visit to the grower may not be necessary at this time. For multi-state traceback investigations, the flow diagrams will be combined and analyzed for common points of origin and distribution. After this final analysis, a farm/source that was initially identified in one traceback may no longer be implicated. See Appendix C for an example of a multi-state traceback flow diagram that illustrates this point.
EOC and CFSAN will evaluate the traceback results along with the epidemiological and laboratory findings and determine if an inspection/investigation is needed at any of the implicated distributors, packinghouses, or growers.
FARM/SOURCE INVESTIGATIONS
Please see the DFI Guide to Produce Farm Investigations, available at http://wcms.fda.gov/FDAgov/ICECI/Inspections/InspectionGuides/default.htm for further guidance.
Attachment 1: Program Assignment Codes for Emergency Responses
| Emergency PAC Code | Title | Problem Area Flag (for sample collection & analysis) |
|---|---|---|
| 03R175 | Cyclospora Emergency | MIC, PAR |
| 03R265 | E. Coli 0157:H7 (EHEC) Emergency | MIC |
| 03R225 | Enterotoxigenic E. coli (ETEC) Emergency | MIC |
| 03R266 | Listeria Emergency | MIC |
| 03R263 | Salmonella Emergency | MIC |
| 03R264 | Salmonella Enteritidis in Eggs Emergency | MIC |
| 03R278 | Scombroid Emergency | MIC |
| 03R224 | Shigella Emergency | MIC |
| 03R236 | Sprouts Emergency | MIC |
| 03R267 | Viral Emergency | MIC |
| 03R277 | Vibrio Emergency | MIC |
| 03R839 | Foodborne Emergency - NEC | MIC |
| 04R839 | Pesticides and Chemical Contaminate – NEC | PES, ELE, CDW |
| 07R279 | Biotoxin Emergency | BIO |
| 07R280 | Alflatoxin/Mycotoxin Emergency | BIO |
| 07R839 | Molecular and Natural Toxin Emergency - NEC | BIO |
| 09R839 | Food and Color Additive Emergency – NEC | FAD, COL |
| 21R281 | Infant Formula Emergency | NIF, MIC, PES, ELE |
| 21R839 | Food Composition, Standards, Labeling & Economics Emergencies – NEC | NUT, MIC, FAD, PES |
| 71R839 | Post Approval Animal Drugs, Feeds and Devices – NEC | PES, ELE, MIC, MYC |
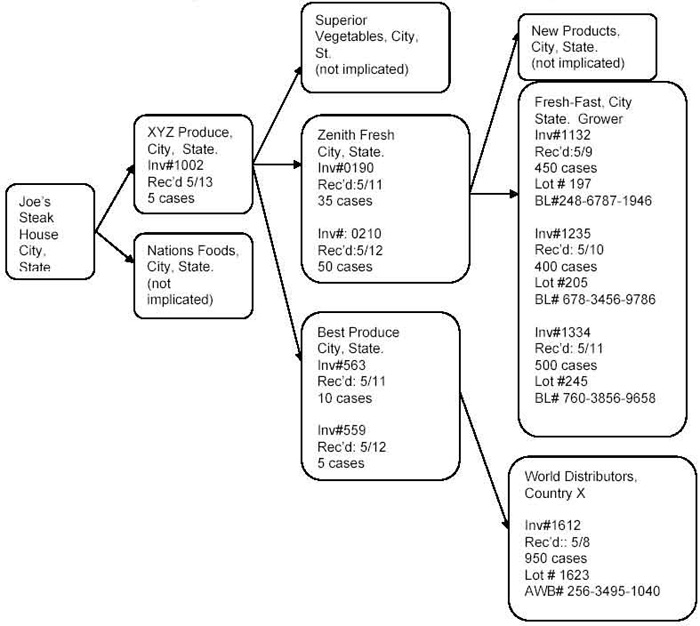
Attachment 2: Traceback Data Analysis Example
Data analysis is conducted with the use of a timeline and a flow diagram. Investigators may choose to develop their own analytical tools. EOC will continue to use the timeline and flow diagram for continuity and sharing of data with other investigators and agencies. If an investigator develops a new tool, they are encouraged to share this with EOC.
TIMELINE
The timeline chart is an easy visual reference that provides information on the receipt dates of deliveries made to each level of distribution of the product implicated in the outbreak, and inventories on any given date, if available. The timeline also illustrates historical use patterns. Analyze data one distribution level at a time, plotting one distributor per line. Use inventories, estimated turnover rates, stock rotation and delivery times to identify suspect product shipments and what distributors need to be visited to continue the traceback. Only exclude shipment or distributor investigations if analysis clearly shows they are not suspect shipments, and they are well separated in time from any implicated outgoing shipments. It is key that the investigator analyzes the data gathered from each distribution level prior to planning data collection for the next level. Because the timeline is expanded at each distribution level, it serves as a guide for the decision making process for all subsequent levels.
DATA ANALYSIS
The following instructions will use a hypothetical example to illustrate how to use the timeline, flow diagram and information collected from interviews. Use the completed timeline and flow diagram to follow along. In the “DATE” row of the chart, insert the date of the event/purchase under investigation into the right-most column. Fill in the dates in each column beginning from the far right so that one shelf life (10 days) prior to the event/purchase date is covered.
Point-of Service
Information gathered from interviews and observations at the POS:
- The product was prepared for the 5/16 event on the morning of 5/15, prior to receipt of any shipments on 5/15.
- The POS had an inventory system. Inventory was taken early before any product arrived from suppliers, so the inventory does not reflect shipments received on the same day.
- The POS used a First-In-First-Out stock rotation which usually means the shipments overlap with only 2-3 shipments prior to the customer delivery date
- Certain shipments could be eliminated because the delivery times of product from suppliers to the point-of-service were after the suspect product was prepared.
After completing the investigation at the POS, the investigator will want to fill in the timeline and analyze the POS information.
- Label the second row “At Joe’s Steak House (the POS), Daily inventory.” If an inventory is not available, indicate (no inventory). If the inventory was not done on a daily basis, only fill in the days for which there is inventory data available and leave the other days blank. Do not write zero if inventory was not taken. Zero means that there was not any product available on site (this includes partial cases, carry over, etc).
- The POS in this example was supplied by two distributors (XYZ Produce, Nations Foods) during the 10-day shelf life. On the next two lines fill in “From XYZ Produce” and “From Nations Food.” Now add the number of shipping unit(s) (the product in this example is shipped in cases) received on each day from each supplier. For example, at Joe’s on 5/16 there was no shipment received from XYZ Produce. Two cases were received on 5/16 from Nations Foods. Remember from the data collection section that it is critical that the investigator clarifies the type of date gathered from the documents and adjusts it to best approximate the receipt date (and document this process). It is the date the product is received that is the determinant of whether product could, or could not, be utilized or delivered to the next level of distribution.
- Make sure that the amounts of product received are consistent with apparent usage and available inventory numbers (i. e., you can’t sell more than you have). In this example, the POS inventory is taken each morning before business and before supplies are delivered. The inventory indicates that all product delivered on 5/10, 5/11, and 5/12 was depleted before the next inventory was performed. This means that the 2 cases delivered from Nations Food on 5/10 were depleted prior to inventory being taken the morning of 5/11. The 4 cases delivered on 5/11 from XYZ Produce were used at the POS before inventory was taken on 5/12, and the 2 cases arriving on 5/12 were depleted before inventory was taken on 5/13. Partial cases are recorded as 1 case or ½ case. Zero inventory means there was no product available. On 5/13 the inventory indicates that no product was available before the 5 cases were delivered from XYZ Produce. The inventory on 5/14 indicates that 3 cases from these 5 were still in-house after business on 5/13 and 2 cases were in-house from XYZ Produce on 5/15 since no more product was delivered from Nations Foods. The shipment of 4 cases from XYZ Produce on 5/15 would have arrived after the product was prepared for the event. Therefore, the likely shipment used to prepare the meal served at the event was the 5 cases on 5/13 from XYZ Produce. No suspect product from Nations Foods was in-house after 5/11 so, although product was delivered by this distributor during the shelf life of the product, there would be no need to visit the distributor for further information. Analysis at each level, as the data is collected, is thus a more efficient use of the investigators time than simply gathering data without subsequent analysis. In the event that there was no inventory, one would look at the historical usage and include the shipment on 5/12 and possibly on 5/11. Inventory records are very useful and help to eliminate shipments and suppliers. This reduces the number of implicated firms. Therefore if an inventory is available, it is a very important tool for the analysis. Lack of an inventory usually results in a few additional shipments and possibly suppliers being implicated.
Distributor, XYZ Produce
Information gathered from interviews and observations at the Distributor:
- The Distributor, XYZ Produce, does not have an inventory system.
- The firm used a First-In-First-Out stock rotation which usually means the shipments overlap with only 2-3 shipments prior to the customer delivery date.
- Certain shipments could be eliminated because the delivery times of product from suppliers to the distributor were after early morning customer deliveries.
- Start a new row and label it “At XYZ Produce.” There was no inventory available at XYZ Produce. Add the shipment data gathered from invoices and shipping records for each of the three XYZ Produce suppliers, one per row, being sure to approximate “receipt” dates.
- Although there is no inventory, it is clear from the product usage during this time that XYZ Produce would not have had any product from Superior Vegetables in-house at the time of the 5/13 delivery. This is based on a daily usage of approximately 48-50 cases per day and incoming deliveries approximately the same. Therefore, most product is probably turned over in 1-2 days. Product delivered to the POS on 5/13 (5 cases) would have had to be delivered to XYZ Produce on 5/12 or earlier since customer deliveries are made before any incoming shipments arrive. Since there is no inventory specifying that the product from 5/12 was the only product available for shipping on 5/13, the investigator would also implicate shipments from at least the previous day (5/11). There would be no way to differentiate between shipments from Zenith Fresh or Best Produce. Analysis indicates that the likely shipments contributing to the shipment of 5 cases to XYZ Produce were the four shipments in bold (2 on 5/11 and 2 on 5/12).
Distributor, Zenith Fresh
- Start a new row and label it “At Zenith Fresh” indicating that shipment amounts entered for the rows below were determined to be in-house at Zenith Fresh during the shelf life of the product. Add the shipment data gathered from invoices and shipping records for each of the two Zenith Fresh suppliers, one per row, being sure to approximate “receipt” dates.
- Moving backwards at a diagonal through the timeline one can use judgment to ascertain which product shipments might have been implicated. The two shipments made from Zenith Fresh to XYZ were on 5/11 and 5/12. Supplier deliveries on 5/12 or later would not have been in-house for delivery to XYZ Produce on 5/12 or earlier (deliveries to customers are loaded in the early morning before any supplier shipments are received). Therefore, no product from New Products (first shipment during shelf life was on 5/12) would have been implicated and no investigation is warranted at New Products. Average daily usage is approximately 450-500 cases so based on product usage the 5/12 shipment probably originated from the “Fresh-N-Fast” shipments on 5/11 and 5/10 (overlap always included if no specific inventory information is available). The 5/11 shipment probably originated from the “Fresh-N-Fast” shipments on 5/10 and 5/9. Therefore, the three Fresh-N-Fast shipments (5/9, 5/10, 5/11) were likely to have contributed to the implicated shipments. Subsequent investigation determined that Fresh-N-Fast was themarketing entity for distributing product from an out-of-state domestically grown source farm.
Distributor Best Produce
- As before, start a new row and label it “At Best Produce” indicating that shipment amounts entered for the rows below were determined to be in-house at Best Produce during the shelf life of the product. Add the shipment data gathered from invoices and shipping records for the Best Produce supplier, being sure to approximate “receipt” dates.
- The two shipments made from Best Produce to XYZ were on 5/11 and 5/12 and average product usage was approximately 900-950 cases every 4 days. The 5/12 shipment of 900 cases would not have arrived in time to be sent on to XYZ (deliveries to customers are loaded in the early morning before any supplier shipments are received) and usage of 250 cases/day makes it highly unlikely that product from the 5/4 shipment was available. Therefore, the single World Product shipment on 5/8 was implicated in the investigation. Interviews at Best Produce indicated that they were direct importers of foreign produce from World Products in Country X.
Conclusions: The outbreak associated with a 5/15 event at the POS served product that traced back to three shipments of product from Fresh-N-Fast (received 5/9, 5/10, 5/11) that were domestically grown in state F and one shipment imported by Best Produce from World Products in Country X.
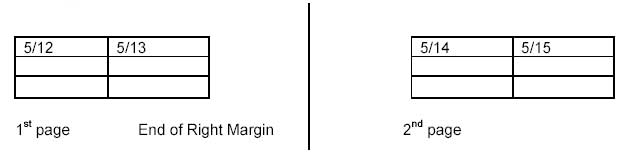
Example of a Completed Time Line
| DATE | 5/06 | 5/07 | 5/08 | 5/09 | 5/10 | 5/11 | 5/12 | 5/13 | 5/14 | 5/15 | 5/16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
At Joe’s Steak House (POS) Daily inventory |
3 | 0 | 4 | 5 | 1 | 0 | 0 | 0 | 3 | 2 | 0 | |
|
From XYZ Produce |
0 | 5 | 0 | 4 | 0 | 4 | 2 | 5 | 0 | 4 | 0 | |
|
From Nations Foods |
2 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | |
| At XYZ Produce no inventory | ||||||||||||
|
From Zenith Fresh |
0 | 40 | 40 | 30 | 25 | 35 | 50 | 0 | 45 | 35 | 35 | |
|
From Best Produce |
0 | 0 | 10 | 5 | 5 | 10 | 5 | 55 | 10 | 10 | 5 | |
|
From Superior Vegetables |
50 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| At Zenith Fresh no inventory | ||||||||||||
|
From New Products |
450 | 500 | 0 | 0 | 0 | 0 | 250 | 300 | 200 | 300 | 0 | |
|
From Fresh-N-Fast Grower- State F |
0 | 0 | 550 | 450 | 400 | 500 | 200 | 300 | 300 | 200 | 450 | |
|
At Best Produce no inventory |
||||||||||||
|
From World Dist. Country X |
0 | 0 | 950 | 0 | 0 | 0 | 900 | 0 | 0 | 0 | 950 | |
Example of a Traceback Investigation Flow Diagram
Flow Diagram
Most traceback investigations resemble a branching tree because of multiple suppliers throughout the distribution chain. An easy way to visualize the ongoing investigation and shipments of product is to draw a flow diagram illustrating each level of the investigation as it branches from the point of service to its original source(s). Prepare a Flow Diagram illustrating distribution of the product up through the distribution level currently under investigation. Include for each implicated distributor: name, city, state, invoice number, receipt date, quantity, lot numbers, and Freight/AWB numbers and dates. For non-implicated distributors list only the distributor name. If there are numerous shipments involved and the flow diagram would become too complex, just list receipt date, quantity, and invoice number on the flow diagram, and include other record information on a separate page.
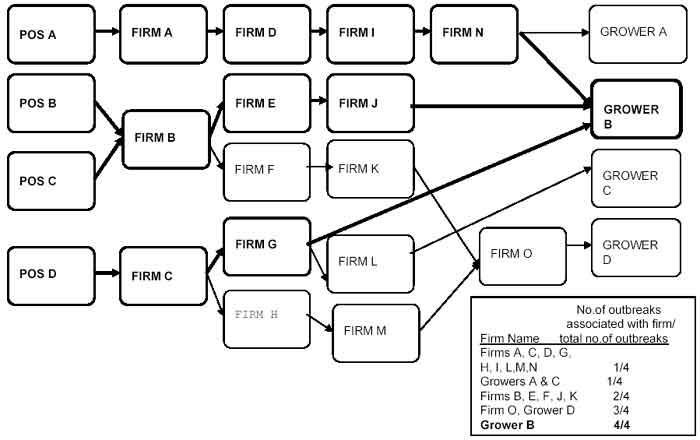
Attachment 3: Multi-state Flow Diagram Example
The boxes and lines that are in bold lettering indicate the implicated product’s distribution pathway that links all of the points-of-service outbreaks to the implicated grower. Tracebacks were initiated at four points-of-service in response to a multi-state outbreak of illnesses that were epidemiologically associated with a food product under FDA jurisdiction. In the traceback that began at POS A, Grower A and B were ultimately implicated. In the tracebacks initiated at POS B and POS C, Growers B and D were both implicated. In the traceback initiated at POS D, Growers B, C and D were implicated. None of the distributors were identified a common supplier to all four points-of-service. Therefore, none of them could have been the source of the contamination. Although a total of four growers were implicated, the only one in common to all of the four tracebacks is Grower B. The only firm that will require an inspection by a team is Grower B.
Attachment 4: Creating Electronic Traceback Documents
Open MS WORD. Select “File.” Select “Open.” Select “Save As.”
Select Save In: Choose a file location (ex. the newly named “event folder.” )
Select File name: give the file a name (example: MI_09flow. doc)
From the top toolbar there should be an icon that has letters and shapes on it. This is the “Drawing” toolbar (place the mouse over the icon the word “Drawing” will appear). Select this icon. A new toolbar will appear at the bottom of the screen.
TO CREATE BOXES
From this new “Drawing” toolbar, select “AutoShapes.” From the pop-up menu, Select “FlowChart.” OR
Select the shape of the box for the diagram (the first two are probably the best choices). They will look like this:

OR

Select the shape and the mouse pointer will become a “ + .” Place this “ + “ where you want the box by clicking once with the left mouse button (it can be moved once it is placed).
TO ADD TEXT TO THE DIAGRAM BOXES
To insert text into the box, place the mouse pointer on the edge of the box and click the Right mouse button. A new menu will appear. Select “Add text.” Type inside the box. Put the name of the firm, the suspect shipment date and quantity. You may choose to change the size of the font before entering the data in the box from the top toolbar. You do not want to use “Text Box” to insert the text. If you do, the text and the box will not be attached and will not move together.
TO CHANGE THE SIZE OF THE BOX
To increase the size of the box, select the box (left mouse click on the box). Place the mouse pointer on one of the small squares outlining the box. You should see a double pointed arrow when it is in position. The end squares will increase the width, the top squares will increase the length and the corner squares will do both. After you have chosen the square, holding the left mouse button down, drag the square outward (or inward) until it reaches the desired size. Release the mouse button.
This box is too small.

This box has been widened and lengthened.

TO MOVE THE BOX
To move the box (and text) to a new position, select the box and place the mouse pointer on an edge of the box until a double arrow appears. Click (and hold) the left mouse button down and drag the box to the desired position. Release the mouse button. You may edit any box anytime by selecting it (left mouse click). To delete an unwanted box simply select it and press delete.
ADDING ARROWS
To add arrows to the flow diagram, select the arrow 
MOVING AND CHANGING ARROWS
To move, shorten or change arrows, simply click on the arrow and a square will appear at each end of the arrow. Using the left mouse button, click and drag these squares to lengthen, shorten or redirect either end of the arrow. To move the entire arrow, place the mouse pointer in the center of the selected arrow and a crossed arrow will appear. Again, left mouse click, hold and drag to new location.
To change the direction of an arrow, select the icon on the “drawing” toolbar that has the 4 arrows lined up horizontally. From there select the arrow direction or type that you want.
To delete an unwanted arrow, simply select it and press delete.
STANDARD ARROW USAGE
Since these flow diagrams will be used to share information between districts and with EOC a standard protocol should be followed. All arrows should point in the direction of the traceback. For example, the arrows should start at the point of service and go in the direction towards the source. For a traceforward (if you are asked to do one), direct the arrows from the source (or starting point of the traceforward) towards the point of service.
HELPFUL HINTS
Each of the levels of the Time Line and Flow Diagram should be consistent with one another. The levels (i.e., to 5A, from 4A) are for illustrative purposes. You will use the name of the Firm. If the diagram does not fit on one page, you will need to indicate where the chart is connected on the next page. (For inserting these instructions, you can use the “text box” option on the “Drawing” toolbar).
Here is an example:
TO ADD A NEW PAGE
Press Ctrl (hold) and END.
Select “Insert” from the top toolbar. Select “New Page.” Be sure to check the “Page Set Up” to see that it is still in Landscape format for this new page.
TO VIEW ENTIRE PAGE
This is helpful to see that all the levels are lined up and the arrows are connected correctly. Select “View.” Select “Zoom.” Select “Whole page.” You will then see the document one page at a time. You may make edits from this view. To change back to the previous page or to another viewing size, Select “View” and choose the size you want.
TIME LINE
TO BEGIN A NEW TIME LINE
Open MS WORD.
Select “File.” Select “Open.” Select “Save As.”
Select Save In: Choose a file location (example: the newly named “event folder.”)
Select File name: give the file a name (example: timeline. doc)
Select “Table” from the toolbar.
Select “Insert Table.”
For number of columns: Choose Shelf Life plus 2 (1 column for event date and 1 for labels).
For number of rows: Begin with 10 and add as needed (as investigation proceeds).
Column width: Auto Format
Select “OK.”
When the time line is complete, be sure to “save” and then it can be submitted electronically via the e-mail system as an attachment.
TO FILL IN THE TIME LINE TABLE
Use the instruction forms provided in this document as to how a time line is completed and analyzed. Remember to analyze at each level using the time line and flow diagram and to consult with EOC before proceeding to the next level. Place the cursor in the desired cell. Type “Date” in the 1st column, 1st Row. Then fill in the dates (the furthest right hand column will contain the event date). The second row will contain the name of the POS and inventory (if kept) on each of the dates. The third row begins the list of distributors who supplied to the POS during the shelf life prior to the event. After these have been listed, the next level will begin. It should start with “at Distributor (A)”, the name of the first supplier to the POS, and continue one row for each of the supplier to Distributor (A). Label these “from Supplier C” (using the name of the firm) for all suppliers to this first distributor. Refer to other information on how to fill out and use a time line.
TO ADD MORE ROWS TO THE TABLE
When there are no more blank rows left in the table, you may need to add more rows.
Place the cursor at the end of the number in the final cell. Press “TAB.” You will now have a new row.
TO CONTINUE A TABLE ON A NEW PAGE
If the table is too long for one page, choose a place to end the table. Try not to split a distributor’s list of suppliers between pages. Select the final row in the table, by placing the cursor on the row. Select “Table.” Select “Select Row.” After highlighting the final row for page 1, Select “Split Table.” This will split the table. However on the new page you will need to label the first row with the dates.
You may choose instead to Select “Insert.” Select “Break.” Select “New page.” On the new page repeat the above instructions for creating a new table, including completely filling out the dates. If the table is too wide (long shelf life), you will need to continue it on a new page, beginning with the next date.
Example:
SUSPECT SHIPMENT IDENTIFICATION ON THE TIME LINE
This table will be transmitted electronically, so you will need to be able to indicate which shipments on the time line are suspect. To do this, you may either put the shipment(s) in bold or shade in the cell containing the shipment(s).
To indicate suspect shipment(s) by BOLD letters simply put the mouse pointer to the left of the first number in the cell and click the left mouse button (and hold) and drag across the numbers you want to be in bold. They will be highlighted. Then from the lowest toolbar at the top of the screen there will be a letter B. Select “B.” The numbers you have highlighted will now be bold.
|
Date |
5/10 |
|---|---|
|
Distributor A |
5 |
|
Distributor B |
6 |
To indicate suspect shipments by SHADING the cell containing the shipment(s), simply highlight the entire cell by pointing the mouse pointer (move it until there is an arrow pointing in the cell) and click the left mouse button. This will highlight the entire cell. Select “Format” from the top toolbar. Select “Borders and Shading.” Select “Shading” tab. From the Patterns/ Style menu you will see “CLEAR” as the selected choice, scroll down until you see 5% or 10% (grey). Select either of these two options. The 10% grey is shown below.
|
Date |
5/10 |
|---|---|
|
Distributor A |
5 |
|
Distributor B |
6 |
Attachment 5: Interview Questions
In the July 1998 Traceback Guide, a questionnaire was completed and included in the final reports. This questionnaire is no longer required. However, many investigators have found these questions useful for eliciting information, so they are included here as a reference.
POS Event Information
- Product Preparation Date/Time (a.m./p.m.):
- Have there been any reports of illness to the POS?
- Were any of your employees ill during the two weeks prior to the event?
Outgoing Deliveries to Customers and Incoming Deliveries from Suppliers
- Can the source of implicated product delivered to customers be tracked precisely by the use of a lot numbering system?
- How are customer deliveries documented or recorded?
- Are the outgoing delivery records initialed or stamped with the delivery date?
- How are supplier deliveries documented or recorded?
- Are the records of an incoming shipment initialed or stamped with the receipt date?
- If the records don’t reflect receipt dates, explain how should the recorded dates be adjusted to reflect receipt dates (for outgoing shipping dates and incoming shipment receipts) ?
- Were there any holidays or unusual occurrences that would have affected delivery or receipt dates?
- What are the transit times from each of the supplier(s) listed above to the current establishment?
- Are there any transfers of products within the company? How are these handled and documented?
Shipping and Receiving Practices
- When and how does the firm order new stock?
- Is there a standard stock “low point” after which the firm orders additional product?
- What happens if the firm does not have enough product to fill an order?
- What are the firm’s stock rotation practices?
- Are the stocking practices generally followed at this establishment? When might they be deviated from?
- Is a stock inventory taken? How often and what time of the day is inventory taken?
- At what time does this establishment load its deliveries to customers?
- If inventory is taken, is it taken before or after deliveries for the day are loaded?
- If inventory is taken, is it taken before or after shipments are received?
- Did the customer(s) pick up the order or was it delivered?
- What are suppliers’ general delivery times?
Product Handling and Storage Practices
- Does the firm have an SOP and documentation for rejected or returned products?
- Does the firm have an SOP for disposal of product too old to sell?
- Is the product prepared, repackaged and/or handled prior to distribution, service, or sale?
- How is the customer product loaded?
- Does the firm mix loads?
- Is there an SOP for loading?
- Does the firm have an SOP for truck cleaning or specifications for acceptance of vehicles for loading?
- Is it clear to the loaders which product should be loaded first?
- What are the approximate shipping times to the firm(s) who received the implicated product?
- How is the incoming product handled upon receipt?
- Do suppliers use any chemical or gas treatment on the product before shipping?
Return to: Page Top | Inspection Start