Drug Development for Very Rare Diseases
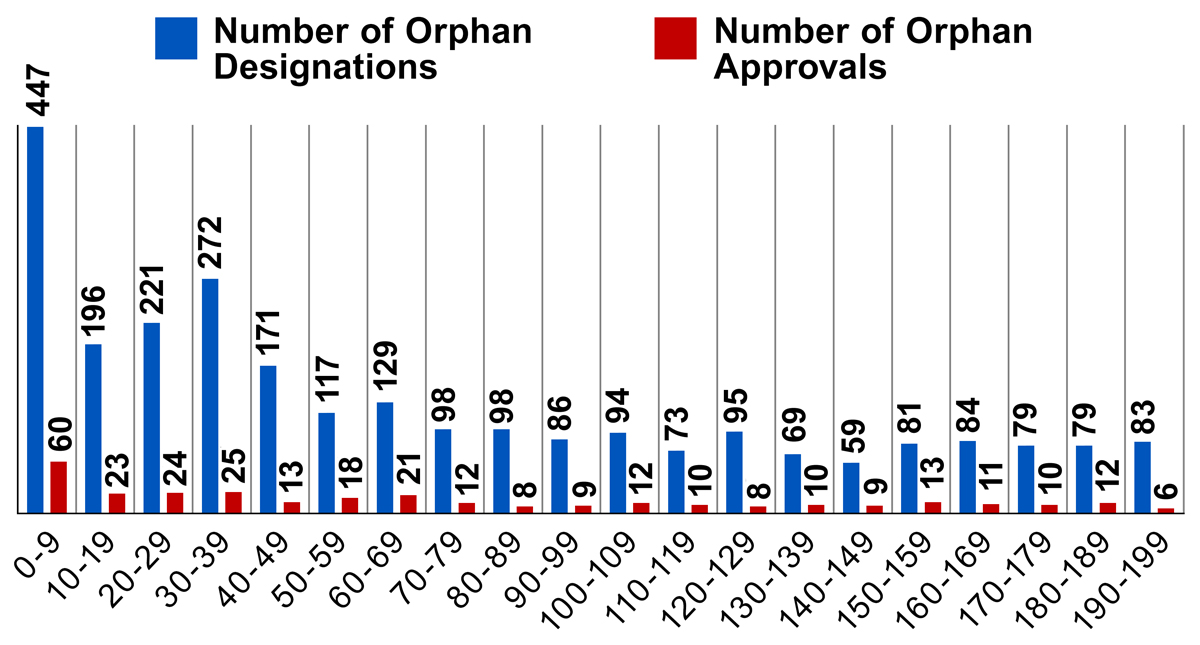
The Orphan Drug Act provides financial incentives to those drugs and biologics designated as orphan drugs. To qualify, these products must be intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the U.S., or that affect more than 200,000 persons but are not expected to recover the costs of developing and marketing a treatment drug. Data shows that most orphan drug designations and approvals from 2008-2017 are for drugs that are intended to treat very rare diseases with a prevalence well below the 200,000 person threshold. The number of orphan drug designations and approvals do not increase if the disease population approaches the 200,000 threshold.