Impact Story: Modeling Tools Could Modernize Generic Drug Development
Quantitative methods and modeling (QMM) covers a broad spectrum of tools that can be used in modernizing generic drug development.1 In both new and generic drug development, mathematical models can be thought of as knowledge management systems that integrate scientific understanding and existing data about drug products. Models can describe an array of characteristics related to drug products, including formulation, in vitro/in vivo release, pharmacokinetics (PK), pharmacodynamics (PD), and clinical responses. The discipline of QMM builds these models and uses them to aid both regulatory and business decisions. QMM can address challenges in bioequivalence (BE) assessment which can potentially provide pivotal information to support regulatory decision making. For both new and generic drugs, QMM can accelerate product development and regulatory assessment. The huge volume of generic drug applications especially translates into numerous opportunities for the use of QMM. The Office of Generic Drugs is currently taking advantage of QMM and is deploying information to industry on best practices for using QMM to modernize generic drug development.
New drug development and approval depends on sufficient in vitro and in vivo evidence to support the regulatory assessment of drug product efficacy and safety. A generic drug is approved based on sufficient demonstration of sameness to the corresponding brand drug.
QMM in New Drug Development
For new drug development, as shown in Figure 1, the data axis (yellow curve) includes information and datasets collected through the full research and development course for the active pharmaceutical ingredient (API), formulation, in vitro release, the targeted in vivo release profile, animal and human drug PK, PD response(s), and clinical responses in terms of both efficacy and safety end points. Models describing the relationships between the datasets are captured in the model axis (green curve). The use of these models can optimize the collection of data and accelerate decisions. The dotted curve represents that combining models and data results in higher confidence in clinical performance at an earlier time in development.
Model-informed drug development (MIDD) under the Prescription Drug User Fee Amendments of 2017 (PDUFA VI) is an initiative to use these models to decrease development uncertainty, cost, time, attritions, and failure rates. It aims to inform drug development and regulatory decisions by using population PK, dose/exposure–response relationships, and biological and statistical models derived from preclinical and clinical data sources. An MIDD pilot program was launched with goals to provide an opportunity for drug developers and FDA to discuss the application of MIDD approaches to the development and regulatory evaluation of medical products in development and provide advice about how particular MIDD approaches can be used in a specific drug development program.2 Some direct benefits of MIDD include reducing the need for additional clinical trials and number of patients for studies, guiding dose and dosing regimen selection, and identifying patient populations.
Figure 1. Model-integrated drug development for new drugs. The x-axis represents the time for product research and development; the y-axis represents the confidence level on the clinical performance of a new drug product. Blue, green, and yellow lines represent model, data, and model integrated evidence processes, respectively, in drug development. Line of model integrated evidence (dashed green line) represents the hidden confidence level behind regulatory decision making. Model integrated evidence from this perspective represents an integration of data and knowledge on quantitative relationships driving clinical outcomes.
QMM in generic drug development
One of the critical elements for approval of generic drugs is BE, defined in 21 CFR § 314.3(b) as “the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.” Guidelines for BE have been well established since the enactment of the Drug Price Competition and Patent Term Restoration Act (Hatch-Waxman Amendments) in 1984.3 Applicants must use the most accurate, sensitive, and reproducible method available to demonstrate the bioavailability (BA) and BE of a product.
In generic drug applications, the BE evidence can include in vitro, PK, PD, and comparative clinical end points. PK provides the most commonly used data to establish BE of drug products; the demonstration of may rely on other types of data when PK-based assessment is not feasible or sufficient. In 2003, the Medicare Prescription Drug, Improvement, and Modernization Act amended section 505(j)(8)(A)(ii) of the Federal Food, Drug, and Cosmetic Act specified that “[f]or a drug that is not intended to be absorbed into the bloodstream, the Secretary may assess bioavailability by scientifically valid measurements intended to reflect the rate and extent to which the active ingredient or therapeutic ingredient becomes available at the site of drug action.”
Many current scientific and regulatory challenges are related to the use of non-PK BE endpoints. For instance, using comparative PD or clinical endpoints can be insensitive to detect formulation difference or costly in terms of time, human capital, and monetary investment. When an in vitro approach is considered, the identification of clinically relevant in vitro testing parameters along with evaluation standards can pose a great scientific challenge. QMM plays a key role in helping both generic drug developers and generic drug reviewers navigate these challenges. QMM can benefit the design of formulation and process, design of BE in vivo study, and innovation on alternative BE assessment pathways.
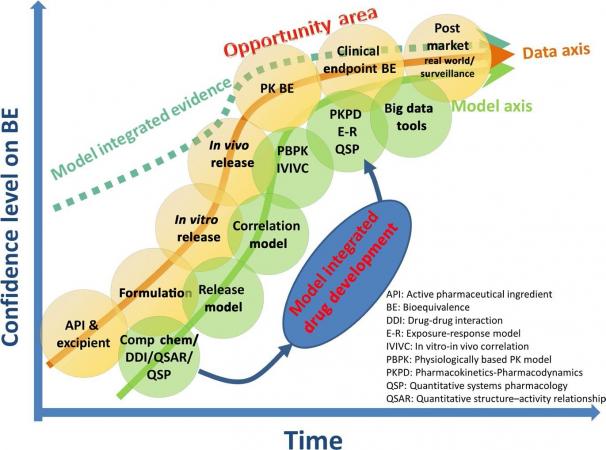
In comparison to the new drug space represented in Figure 1, BE establishment in the generic space (Figure 2) shows three differences in considerations within the data curve: datasets/information for excipients when a new formulation is involved, PK BE, and comparative clinical endpoint BE. Similar to new drug development, when the data collected is combined with a modeling approach the confidence in bioequivalence (dashed line) can reach a higher point at an earlier time. A concrete example is when a modeling approach corroborates in vitro data in the assessment of a bioequivalence.
QMM encompasses in vitro BE methods, identification of clinically relevant quality attributes and specifications, physicochemical (Q3) parameter selection, and in vivo BE standards. All in vivo BE assessment methods are accompanied by quantitative standards to ensure product equivalence in both efficacy and safety. BE standards should be based on a good understanding of the corresponding PK variability and the dynamic PK–PD/exposure–response relationship for the API. We now have examples where QMM has successfully supported a new BE assessment method for drugs having a narrow therapeutic index,4 highly variable drugs,5 and products with partial area under the curve (pAUC) requirements.6
Figure 2. Model-integrated drug development for generic drugs. The x-axis represents the time for product research and development; the y-axis represents confidence on the generic product BE. Opportunity area represents where model integrated evidence has the potential to serve as pivotal information for generic drug approval to replace otherwise required in vivo PK and/or comparative clinical endpoint BE studies and can make in vitro–only BE assessment possible.
QMM plays a role from early to late stages of the life cycle of generic products. FDA aspires to provide practical product-specific guidance, rooted in scientific methods, to guide industry in product development. In cases of complex products without a guidance (owing to knowledge gaps or applicant desires to pursue an alternative BE approaches not otherwise covered in product-specific recommendations) potential applicants can take advantage of the pre-ANDA (abbreviated NDA) program to seek FDA advice.7 Pre-ANDA meetings are designed to clarify regulatory expectations for prospective applicants early in product development, help applicants develop more complete submissions, and promote a more efficient and effective review process. The use of QMM is becoming a more common and important element in pre-ANDA meetings.
The next step for QMM can be to use MIE that provides pivotal information for generic drug approval or, in combination with relevant in vitro BE assessment, serve as an alternative to conducting costly, time consuming, and less sensitive in vivo studies in appropriate cases. Accompanied with PBPK, QCP, or big data–based method developments, virtual bioequivalence (VBE) simulations are closer to becoming standard. The scope of application of VBE simulations depends on data and information available to qualify and verify the underlying model and its use for the intended purpose. Through VBE simulations, MIE can be generated to assist regulatory decision making. Promoting future QMM applications involves stakeholders from FDA and the generic drug industry. Scientists in FDA and industry should form an ecosystem to modernize the generic value chain by leveraging QMM in pre-ANDA interactions and ANDA submission packages to shorten development timelines and reduce costs. All stakeholders should take full advantage of emerging tools, like big data analytics, to aid product development, regulatory review, and postmarketing evaluation.
[1] Journal article “Generating Model Integrated Evidence for Generic Drug Development and Assessment,” by Liang Zhao, Myong-Jin Kim, Lei Zhang and Robert Lionberger, published in Clinical Pharmacology & Therapeutics, Volume 105, Number 2, February 2019.
[2] U.S. Food and Drug Administration. Model-informed drug development pilot program https://www.fda.gov/drugs/development-resources/model-informed-drug-development-pilot-program (August 2018). Accessed April 9, 2020.
[3] U.S. Food and Drug Administration. Draft guidance for industry: Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioequivalence-studies-pharmacokinetic-endpoints-drugs-submitted-under-abbreviated-new-drug (December 2013). Accessed April 9, 2020; U.S. Food and Drug Administration. Draft guidance for industry: Bioavailability Studies Submitted in NDAs or INDs – General Considerations https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioavailability-studies-submitted-ndas-or-inds-general-considerations (February 2019). Accessed April 9, 2020.
[4] Jiang, W. et al. A bioequivalence approach for generic narrow therapeutic index drugs: evaluation of the reference-scaled approach and variability comparison criterion. AAPS J. 17, 891–901 (2015).
[5] U.S. Food and Drug Administration. Draft guidance for industry: Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioequivalence-studies-pharmacokinetic-endpoints-drugs-submitted-under-abbreviated-new-drug (December 2013). Accessed April 9, 2020.
[6] Lionberger, R.A., Raw, A.S., Kim, S.H., Zhang, X. & Yu, L.X. Use of partial AUC to demonstrate bioequivalence of Zolpidem Tartrate Extended Release formulations. Pharm. Res. 29, 1110–1120 (2012).
[7] U.S. Food and Drug Administration. Draft guidance for industry: Formal Meetings Between FDA and ANDA Applicants of Complex Products Under GDUFA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/formal-meetings-between-fda-and-anda-applicants-complex-products-under-gdufa-guidance-industry (October 2017). Accessed April 9, 2020.