2023 Scientific Computing Days - Poster Gallery
Poster Gallery
| Watch Video! | Poster PDF | Title | Authors | Contact |
|---|---|---|---|---|
 (click to watch!) |
 |
Bayesian Spatial Cluster Signal Learning with Application to Adverse Event (AE) | Hou-Cheng Yang | Hou-Cheng.Yang@fda.hhs.gov |
 (click to watch!) |
 |
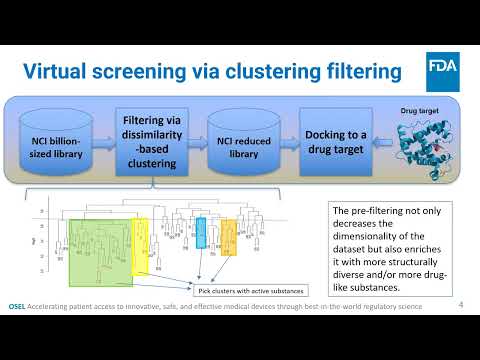
Scalable Filtering of Chemical Substances on HPC Clusters | Mike Mikailov, Yulia Borodina, Fu-Jyh Luo, Kenny Cha | Mike.Mikailov@fda.hhs.gov |
 (click to watch!) |
 |
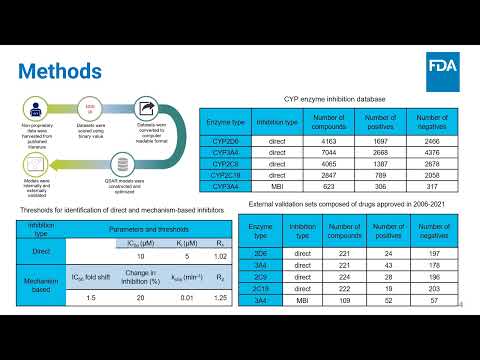
Novel 2D QSAR Models for Prediction of Reversible and Time-Dependent Inhibition of Cytochrome P450 Enzymes by Small Molecules | Sadegh Faramarzi Ganj Abad, Lidiya Stavitskaya, Donna Volpe, Xinning Yang | Sadegh.faramarziganjabad@fda.hhs.gov |
 (click to watch!) |
 |
Using Information Theory to Find Codon and Codon Pair Bias Networks | Nathan Clement, Nobuko Katagiri, Chava Kimchi-Sarfaty | Chava.Kimchi-Sarfaty@fda.hhs.gov |
 (click to watch!) |
 |
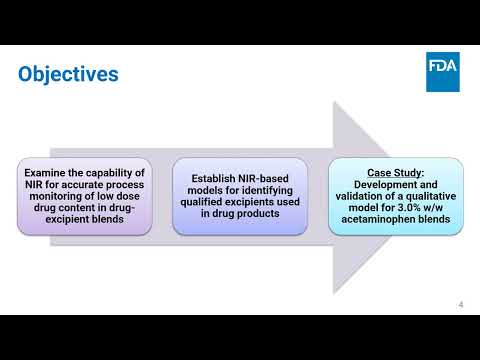
Evaluation of Near Infrared (NIR) for the Qualitative Detection of Low Dose Drugs | Scott Krull, Tao Ding, Xiaoming Xu | Scott.Krull@fda.hhs.gov |
 (click to watch!) |
 |
Towards Cognitive Human Digital Twins for Simulations and Collaborations on the Metaverse | Tam Nguyen | Tam.Nguyen@fda.hhs.gov |
 (click to watch!) |
 |
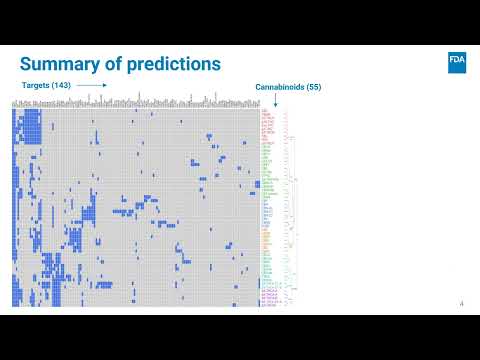
Predicting Binding Between 55 Cannabinoids and 4799 Biological Targets by In Silico Methods | Michael Santillo, Robert L. | Michael.Santilla@fda.hhs.gov |
 (click to watch!) |
 |
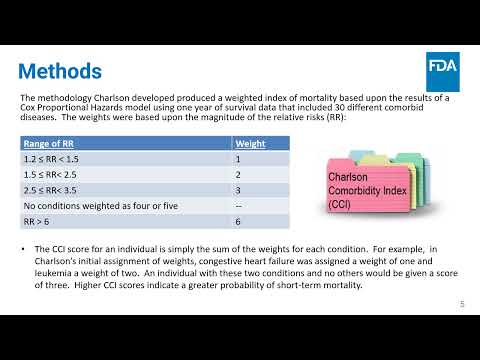
Calibrating a Charlson Comorbidity Index for the American Indian Population Using the Results from the Strong Heart Study | Paul Rogers, Christine Merenda, Richardae Araojo, Christine Lee, Milena Lolic, Dong Wang, Wen Zou, Joshua Xu | Paul.Rogers@fda.hhs.gov |
 (click to watch!) |
 |
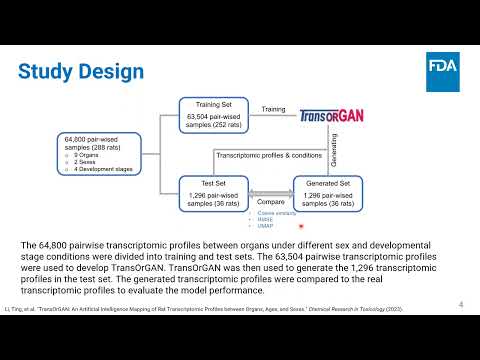
TransOrGAN: An Artificial Intelligence Mapping of Rat Transcriptomic Profiles between Organs, Ages, and Sexes | Ting Li, Weida Tong | Ting.Li@fda.hhs.gov |
 (click to watch!) |
 |
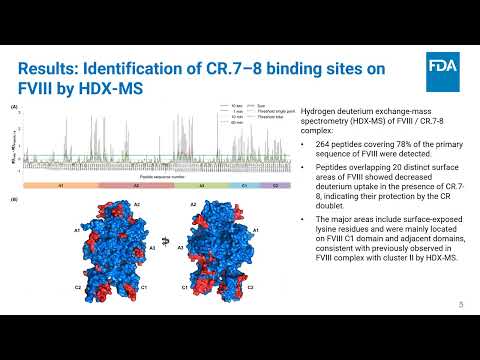
Characterization of FVIII Binding Sites for LRP1 Using Hydrogen-Deuterium Exchange Mass Spectrometry and In Silico Docking | Haarin Chun, Andrey Sarafanov | Haarin.Chun@fda.hhs.gov |
 (click to watch!) |
 |
Comparing the Accuracy of Diagnostic Tests in Studies with Extreme Verification Bias: A Bayesian Model and Gibbs Sampling Computing Algorithm | Ty Ngoc Nguyen, Gene Pennello | NgocTy.Nguyen@fda.hhs.gov |
 (click to watch!) |
 |
AI-Assisted Tool to Improve the Quality and Assessment of PLGA Formulations | Jayanti Das, Md Easin Hasan, William Smith, Qin Bin, Wang Yan, Tian Geng, Xiaoming Xu | Jayanti.Das@fda.hhs.gov |
 (click to watch!) |
 |
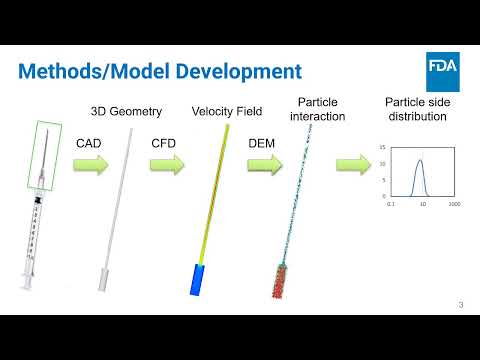
A Machine Learning Approach for Predicting Hemophilia A Severity | Atul Rawal, Christopher Kidchob, Ou (Kelly) Jiayi, Osman Yogurtcu, Hong Yang, Zuben Sauna | Atul.Rawal@fda.hhs.gov |
 (click to watch!) |
 |
Impact of Shear Rate on the Flocculation State and Dissolution of Injectable Suspensions: A Numerical Study | Jianan Zhao, Geng Tian, William Smith, Xiaoming Xu | Jianan.Zhao@fda.hhs.gov |
 (click to watch!) |
 |
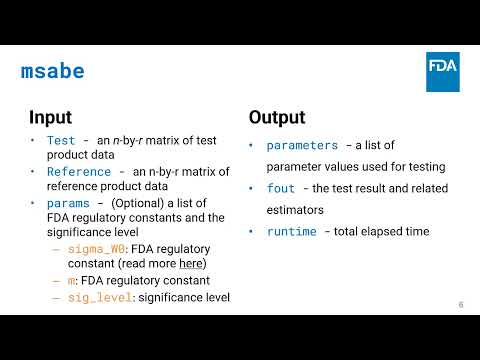
Developing Web Applications for Expedited Risk Assessment at the FDA | Hong Yang, Yin Huang, Rebecca Kahn, Jason Claeys, Mark O. Walderhaug, Wei Wang, Richard Forshee | Rebecca.Kahn@fda.hhs.gov |
 (click to watch!) |
 |
'adaptIVPT' an R-package for Assessing Bioequivalence of Topical, Dermatological Products via an Adaptive Design | Nam Hee Choi, Sungwoo Choi, Elena Rantou | Elena.Rantou@fda.hhs.gov |
 (click to watch!) |
 |
PrecisionFDA Crowdsourcing Addresses Veteran Health Needs While Advancing the Science of Real-World Data (RWD) | Anish Prasanna, Sarah Prezek, Vishal Thovarai, Ezekiel Maier, Adrienne Phifer, Elaine Johanson | Anish.Prasanna@fda.hhs.gov |
 (click to watch!) |
 |
Scientific Review Alignment and Knowledge Gap Analysis in Data Multiverse | Chetan Paul | Chetan.paul@fda.hhs.gov |
 (click to watch!) |
 |
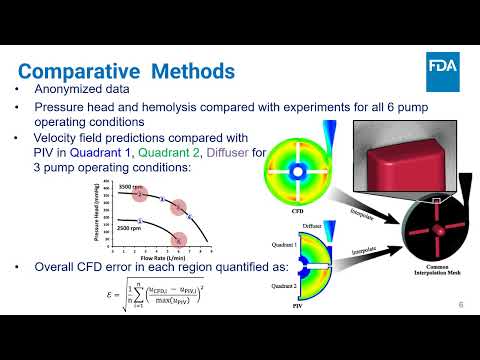
Comparison of Interlaboratory Computational Simulations of Flow and Blood Damage in the FDA Benchmark Blood Pump | Sailahari Ponnaluri, Prasanna Hariharan, Luke H. Herbertson, Richard A. Malinauskas, Brent Craven | Sailahari.Ponnaluri@fda.hhs.gov |
 (click to watch!) |
 |
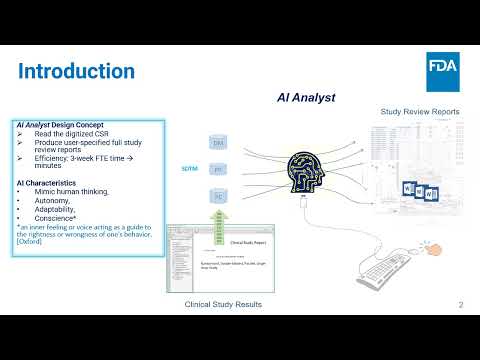
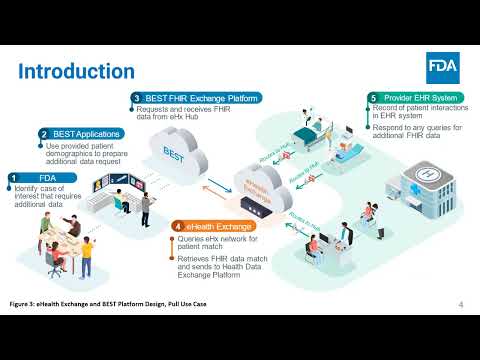
Add Conscience to a Generative AI Platform, Achieving Responsible AI for Clinical R&D Ider | Peter Lee, Le Wang, Gunjan Gugale, Urvashi Somani, Md Nayeem Hossain | Ider.Lee@fda.hhs.gov |
 (click to watch!) |
 |
Enhancing Biologics Adverse Event Surveillance via Scalable, FHIR-based Infrastructure: Pilot | Hussein Ezzeldin, Matthew Deady, Steven Anderson, Lance Jones, Aaron Walerysiak, Sofia Aschettino | Hussein.Ezzeldin@fda.hhs.gov |
 (click to watch!) |
 |
A Systematic Analysis and Data Mining of Opioid-Related Adverse Events Submitted to the FAERS Database | Wen Zou, Huyen Le, Ru Chen, Beverly Lyn-Cook, Huixiao Hong, Paul Rogers, Weigong Ge, Weida Tong | Wen.Zou@fda.hhs.gov |
 (click to watch!) |
 |
AI-guided Experimental Summarization and Sentiment Analysis of Docket Comments | Rahul Paul, Arya Eskandarian, Luis Santana-Quintero, Kevin Kho, Harinder Chahal | Rahul.Paul@fda.hhs.gov |
 (click to watch!) |
 |
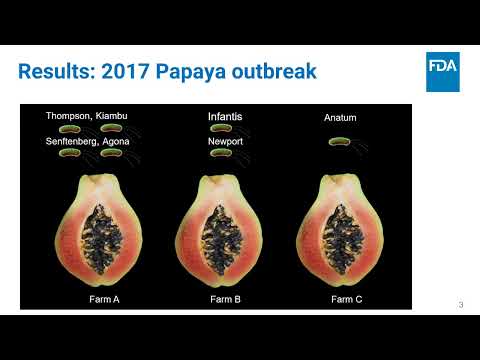
Leveraging FDA’s GenomeTrakr Data with Visualization Dashboards | Maria Balkey, Marc Allard, Tina Pfefer, Candace Bias, Ruth Timme | Maria.Balkey@fda.hhs.gov |
 (click to watch!) |
 |
Dashboard for Interactive Analysis of Mycotoxin Occurrence in Human Foods Data: Streamlining Annual Report Generation and Data-Sharing | Tabitha Miller, Anthony Adeuya, Ernest Kwegyir-Afful, Lauren (Posnick) Robin | Tabitha.Miller@fda.hhs.gov |
 (click to watch!) |
 |
Evaluation of Digital Biomarkers in Nonclinical Research: Barriers to Information Sharing and Collaboration | John Dennis | John.Dennis@fda.hhs.gov |
 (click to watch!) |
 |
bettercallsal: Better Calling of Salmonella Serotypes from Enrichment Cultures Using Shotgun Metagenomic Profiling and its Application in an Outbreak Setting | Padmini Ramachandran | Padmini.Ramachandran@fda.hhs.gov |
 (click to watch!) |
 |
In-silico Study of the Effect of Including Lactose Fines in Modeling Dry Powder Inhaler Performance | Jae Lee | Jae.Lee@fda.hhs.gov |
 (click to watch!) |
 |
Data Central: Accelerating CDER’s Regulatory Review Process by Providing Quality Study Data at the Right Time, in the Right Place, in the Right Format | Slyvester Ezeani, Maren Savignano, James Wedge, Matthew Wiley, Ketan Deopujari, Omolola Ajala, Matthew Robinson, Austin Ford, Jack Slattery, Sofiya Bandura, Sabrina Baldassarre, Christine Cushwa | James.Wedge@fda.hhs.gov |
 (click to watch!) |
 |
Automating Active Surveillance of Online Health Fraud using Artificial Intelligence | Benjamin Ballintyn | Benjamin.Ballintyn@fda.hhs.gov |
 (click to watch!) |
 |
Updates to BioCompute Tools and Guidelines for Use | Charles King | Charles.King@fda.hhs.gov |
 (click to watch!) |
 |
OCS Analysis Studio | Shraddha Thakkar | Shraddha.Thakkar@fda.hhs.gov |
 (click to watch!) |
 |
Governing the Multiverse: Implementing Data Governance to Enhance Data Sharing and Collaboration in the Evolving and Expanding Landscape of CTP’s Data Multiverse | Jack Slattery | John.Slattery@fda.hhs.gov |

