2022 Scientific Computing Days - Poster Gallery
Poster contest opened Mon., 8/8 at 12PM ET and closed Fri., 8/19 at 3PM ET.
Poster Contestants
| Watch Video! | Poster PDF | Title | Authors | Contact |
|---|---|---|---|---|
 (click to watch!) |  | A Metagenomic Analysis for Combination Therapy of Multiple Classes of Antibiotics on the Prevention of the Spread of Antibiotic Resistant Genes | Matthew Igo, Lei Xu, Ashok Krishna, Sharron Stewart, Lin Xu, Zhihua Li, Barry Rosenzweig, James Weaver, Heather Stone, Leonard Sacks, Jeffry Florian, Xiaomei Han, Rodney Rouse | Matthew.Igo@fda.hhs.gov |
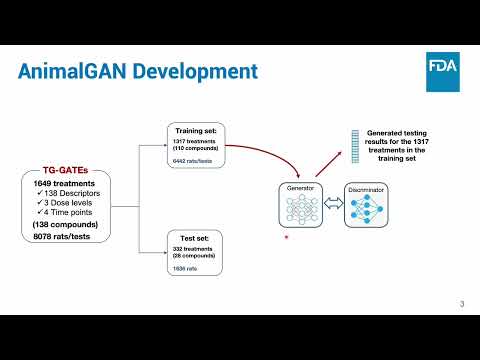 (click to watch!) | 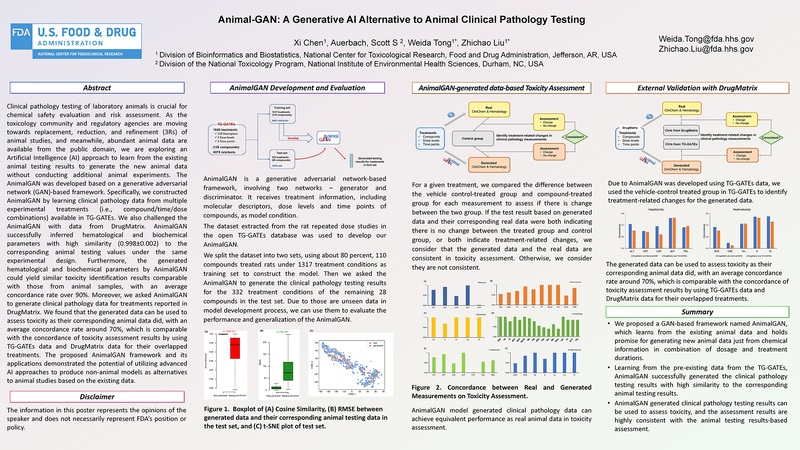 | AnimalGAN: A Generative AI Alternative to Animal Clinical Pathology Testing | Xi Chen, Weida Tong, Zhichao Liu, Scott Auerbach | Xi.Chen@fda.hhs.gov |
 (click to watch!) |  | Assessing Residence Time Distributions and Hold-up Mass in Continuous Powder Blending using Discrete Element Method | Geng Tian, Wei Yang, Scott Krull, Naresh Pavurala, Xiaoming Xu, Thomas O'Connor | Geng.Tian@fda.hhs.gov |
 (click to watch!) |  | Classifying Unformatted Texts into Organized Sections Using BERT Language Modeling | Magnus Gray, Joshua Xu, Leihong Wu | Leihong.Wu@fda.hhs.gov |
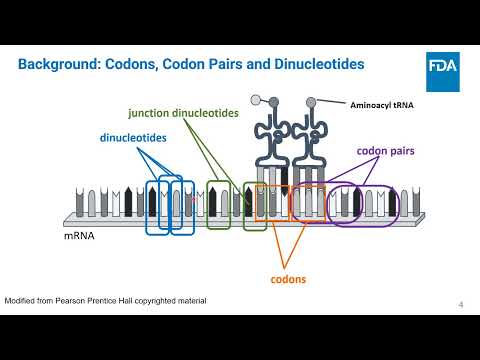 (click to watch!) |  | Creating new and updated codon usage tables in HIVE using species-specific genomic and tissue-specific transcriptomic information | Luis Santana-Quintero, Douglas Meyer, Sean Smith, Marianna Faradzheva, Anton Golikov, Chava Kimchi-Sarfaty, Konstantinos Karagiannis, John Athey, Aikaterini Alexaki, Jacob Kames | Luis.Santana-Quintero@fda.hhs.gov |
 (click to watch!) |  | BRisk and GREAT: Rapid Risk Assessment for Transfusion-Transmitted Infectious Diseases on AWS (Amazon Web Services) | Hong Yang, Yin Huang, Rebecca Kahn, Jason Claeys, Leslie Eberhardt, Mark Walderhaug, Wei Wang, Richard Forshee | Rebecca.Kahn@fda.hhs.gov |
 (click to watch!) |  | Discounting Effect Size when Borrowing Prior Data | Zhuanzhuan Ma | Xuenfeng.Li@fda.hhs.gov |
 (click to watch!) |  | Finding microRNA markers in the African Green Monkey Kidney cells model of neoplastic transformation | Ilya Mazo, Luis Santana-Quintero, Andrew M. Lewis, Daniel Rotroff, Gideon Foseh, Keith Peden, Lauren Brinster | Ilya.Mazo@fda.hhs.gov |
 (click to watch!) |  | Insights into molecular recognition and residence times of opioids in the µ-opioid receptor from molecular dynamics simulation | Paween Mahinthichaichan, Lidiya Stavitskaya, Christopher Ellis; Jana Shen | Paween.Mahinthichaichan@fda.hhs.gov |
 (click to watch!) | 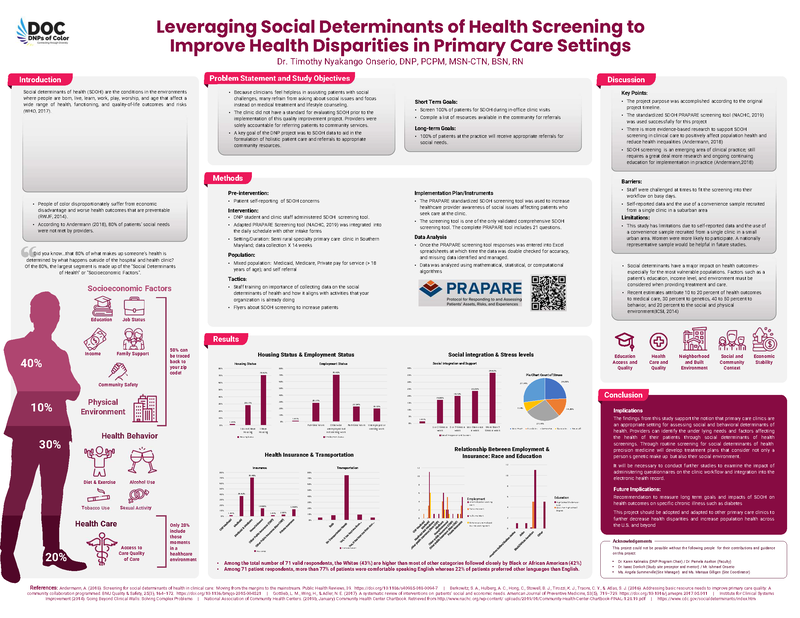 | Leveraging Social Determinants of Health Screening to Improve Health Disparities in Primary Care Settings | Timothy Onserio | Timothy.Onserio@fda.hhs.gov |
 (click to watch!) |  | Leveraging the precisionFDA bioinformatics platform to support developers | Alexis Norris, Carlo Jose Mercado, Adam Moyer, Heather Lombardi | Alexis.Norris@fda.hhs.gov |
 (click to watch!) |  | LexMapr2, an LexMapr update to retrieve current ontology information | Kayla Pennerman, Maria Balkey, Ruth Timme | Ruth.Timme@fda.hhs.gov |
 (click to watch!) |  | Medical device reports indicate some devices associated with higher occurrence of adverse events in women | Tsung-Jen Liao, Lynn Crosby, Gwendolyn Halford, Minjun Chen, Rosalie Elespuru, Kevin Cross | Rosalie.Elespuru@fda.hhs.gov |
 (click to watch!) |  | Medical Information Mart for Intensive Care (MIMIC-III): A Real-World Data Foundation for Reproducible Artificial Intelligence Machine Learning Research | Paul Rogers, Dong Wang, Zhiyuan Lu | Paul.Rogers@fda.hhs.gov |
 (click to watch!) |  | Platform Support for the “BioCompute” Standard, IEEE 2791-2020, and a system for transmitting BioCompute Objects to the FDA | Jonathon Keeney, Charles King, Omar Serang, Samuel Westreich, Anton Golikov, Konstantinos Karagiannis, Mark O Walderhaug, Dennis Dean, Steven Bridges, Phil Webster, Tianyi Wang, Raja Mazumder | Charles.King@fda.hhs.gov |
 (click to watch!) |  | precisionFDA Truth Challenge V2: Using Crowdsourcing to Benchmark Variant Calling Innovation | Elaine Johanson, Ezekiel Maier, Nathan Olson, Justin Wagner, Jennifer McDaniel, Justin Zook, Samuel Westreich, Omar Serang, Anish Prasanna | Elaine.Johanson@fda.hhs.gov |
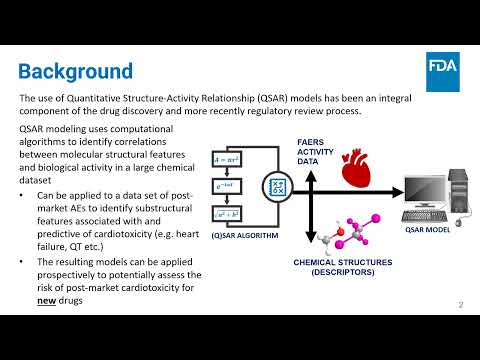 (click to watch!) |  | Quantitative Structure-Activity Relationship Model to Predict Cardiac Adverse Effects | Zhongyu Mou, Rebecca Racz, Lidiya Stavitskaya, Kevin Cross, Mounika Girireddy, Suman Chakravarti | Lidiya.Stavitskaya@fda.hhs.gov |
 (click to watch!) | 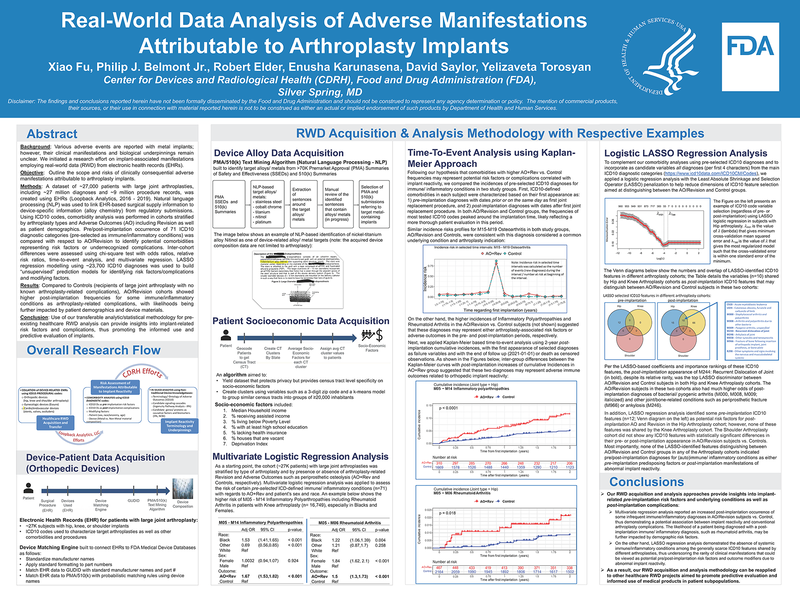 | Real-World Data Analysis of Adverse Manifestations Attributable to Arthroplasty Implants | Xiao Fu, Philip Belmont, Robert Elder, Enusha Karunasena, David Saylor, Yelizaveta Torosyan | Yelizaveta.Torosyan@fda.hhs.gov |
 (click to watch!) |  | Scaling Cheminformatics Computational Simulations on HPC Clusters | Mike Mikailov, Yulia Borodina, Fu-Jyh Luo, Stuart Barkley, Kenny Cha, Nadya Tarasova, Marc C. Nicklaus | Mike.Mikailov@fda.hhs.gov |
 (click to watch!) | 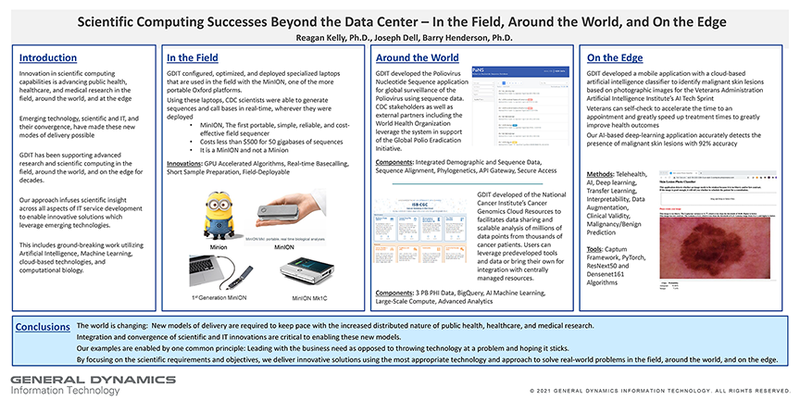 | Scientific Computing Successes Beyond the Data Center | Reagan Kelly, Joseph Dell, Barry Henderson | Kishore.Suresh@fda.hhs.gov |
 (click to watch!) |  | Screening biological products for antibiotic resistant genes using the HIVE ABR pipeline | Sean Smith, Luis Santana-Quintero, Paul Carlson, Konstantinos Karagiannis | Konstantinos.Karagiannis@fda.hhs.gov |
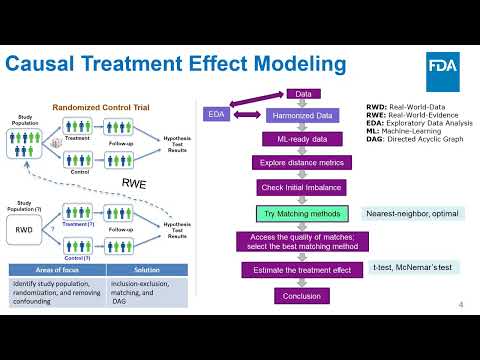 (click to watch!) |  | Simulating In-Silico Clinical Research using diverse Real-World Data | Ryan Weil, Angela Carrigan, Sarangan Ravichandran | Chetan.Paul@fda.hhs.gov |
 (click to watch!) |  | The Adaptability of using AI for Drug Safety Assessments within Regulatory Science: A Case Study of DeepDILI | Skylar Connor, Ting Li, Zhichao Liu, Weida Tong | Skylar.Connor@fda.hhs.gov |
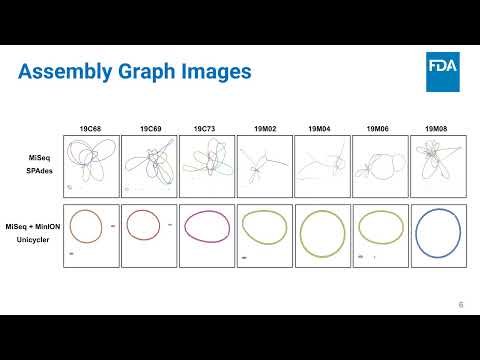 (click to watch!) |  | Two are Better Than One: Hybrid Genome Assembly using MiSeq and MinION | Jason Neal-McKinney, Wen-Hsin (Cindy) Wu, Jinxin Hu | Jason.Neal-McKinney@fda.hhs.gov |
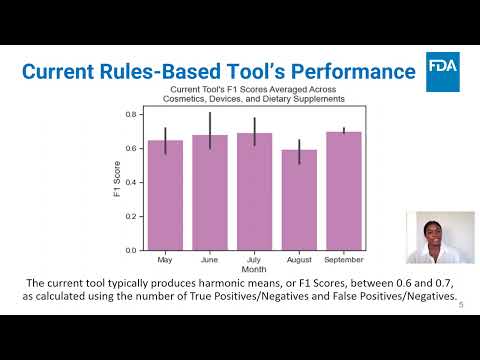 (click to watch!) |  | Using Artificial Intelligence and Machine Learning to Modernize and Optimize the Imports Screening Process | Camryn Bishop, Amanda Groccia, Indu Konduri, Alexander Hanson, Jacqueline Guill, Gregory Parcover, Aisling Casey, Jason Sadler | Indu.Konduri@fda.hhs.gov |

