IND Application Procedures: Exemptions from IND Requirements
Not all investigator-initiated clinical investigations require an Investigational New Drug (IND) application. Before contacting FDA or submitting an IND for an investigator-initiated clinical investigation, investigators should review the exemption criteria in the Code of Federal Regulations (CFR) at 21 CFR 312.2 and refer to the Guidance for Clinical Investigators, Sponsors, and IRBs: Investigational New Drug Applications (INDs)— Determining Whether Human Research Studies Can Be Conducted Without an IND to determine whether their clinical investigations requires submission of an IND application.
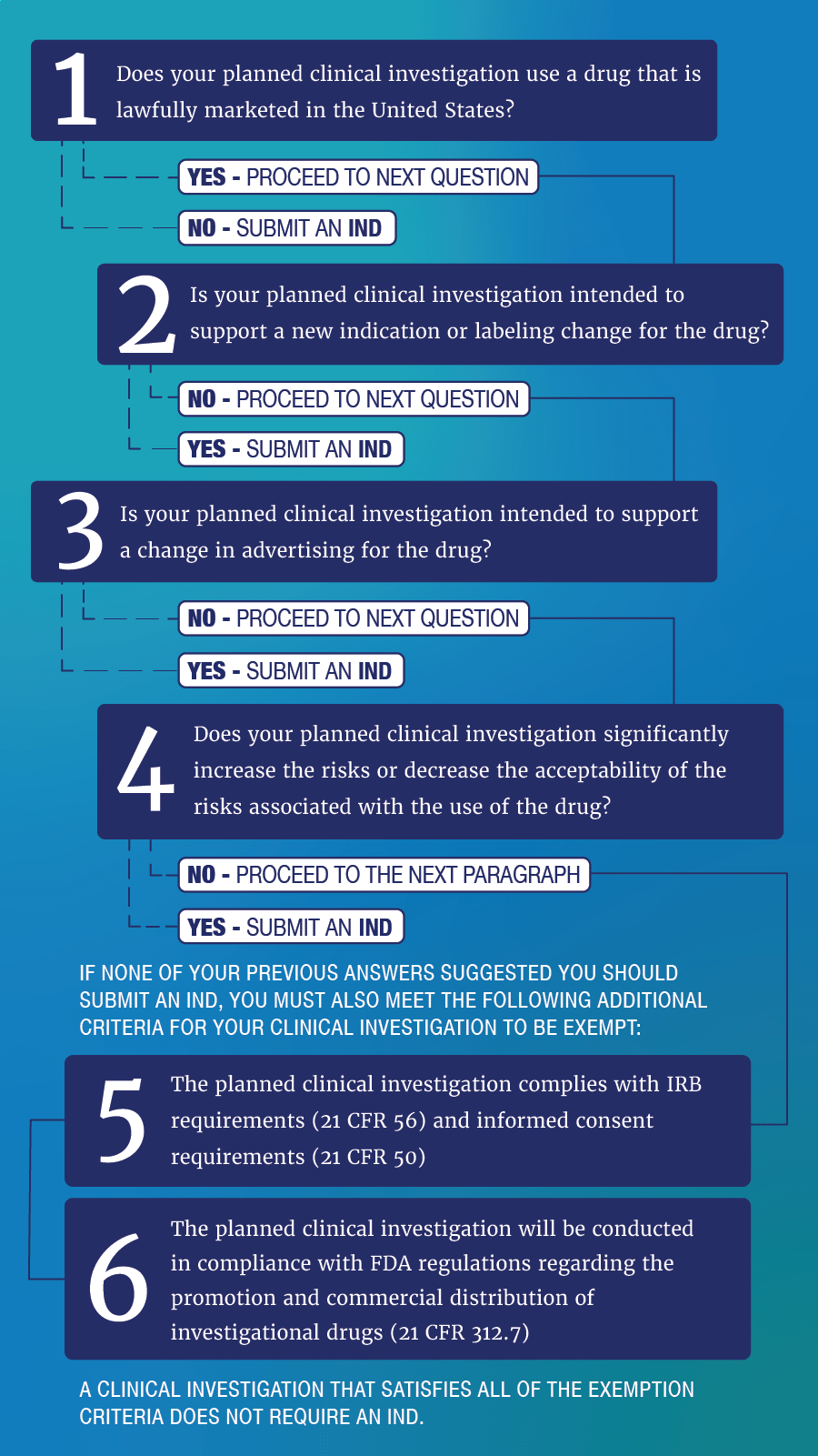
Generally, a clinical investigation of a marketed drug is exempt from the IND requirements if all of the criteria for an exemption under 21 CFR 312.2(b)(1) are met:
- The drug product is lawfully marketed in the United States
- The clinical investigation is not intended to be reported to FDA as a well-controlled study in support of a new indication (new FDA-approved use) for the drug and there is no intent to use the clinical investigation to support any other significant change in the labeling of the drug
- In the case of a prescription drug, the clinical investigation is not intended to support a significant change in the advertising for the drug
- The clinical investigation does not involve a route of administration, dose, patient population, or other factor that significantly increases the risk (or decreases the acceptability of the risk) associated with the use of the drug
- The clinical investigation is conducted in compliance with Institutional Review Board (IRB) requirements (21 CFR part 56) and with the requirements for informed consent (21 CFR part 50)
- The investigation is conducted in compliance with 21 CFR 312.7 (generally prohibiting the commercial marketing or promotion of investigational drugs)
Investigators may use the IND Exemption Decision Tree below as an educational tool for help in determining whether a proposed clinical investigation is IND exempt or requires an IND.
IND Exemption Decision Tree *
* This decision tree is an educational tool designed to assist you in applying FDA's IND exemption criteria under 21 CFR 312.2(b)(1) and determining whether your clinical investigation is IND-exempt. The decision tree is not an FDA determination that a particular clinical investigation is or is not exempt from the IND requirements at 21 CFR part 312. The FDA reserves all rights to verify the status of a clinical investigation and take action as appropriate under applicable law.
In most cases, FDA believes that investigators and IRBs will be able to assess whether the exemption criteria are applicable to the proposed clinical investigation without FDA input. FDA is aware that some investigators and IRBs are requiring IND exempt letters from FDA as a routine practice even where there is no uncertainty in applying the 312.2 exemption criteria to the proposed clinical investigation. FDA exemption letters are not required for exempt clinical investigations to proceed. To ensure the efficient use of FDA resources, investigators and IRBs should not require FDA exemption letters as a routine practice where there is no ambiguity in applying the exemption criteria to the proposed clinical investigation. Investigators should not submit template letters asking for an IND exempt letter either informally or by filing an IND with an exemption letter request.
If seeking FDA advice in applying a specific exemption criterion to the proposed clinical investigation, an investigator should submit questions to the CDER NextGen portal, which will assign a Pre-IND number for FDA correspondence. Investigators should not submit an IND application with an FDA Form 1571 when seeking such advice.
When submitting an exemption question to the portal, the investigator should identify the specific exemption criterion for which there is uncertainty, include a copy of their proposed protocol, and identify the specific aspects of the protocol that create such uncertainty. In response, FDA will notify the investigator that the proposed clinical investigation meets the criteria for exemption under 312.2 or advise the investigator to submit a full IND application.