FDA Facts: Postmarket Patient Registry Ensures Access to Safe and Effective Devices
The U.S. Food and Drug Administration uses flexible trial designs and postmarket data gathering to speed certain new drugs and devices to market. Registries—real-world databases that can be used to track how patients respond to particular medical devices—are one useful source. After products are FDA cleared or approved, and after patients use them or have them implanted, device registries collect information such as how an implantation method affects outcomes or how a device performs over time. The linking of registry data to other data sources such as medical claims also allows for effective long-term monitoring. Registries support the FDA’s ability to learn about potential problems (a process called “device surveillance”) and can therefore improve patient outcomes. They also encourage innovation by allowing manufacturers to collect data more efficiently than via traditional clinical studies, thereby saving manufacturers’ time and money.
The FDA’s work with a patient registry for transcatheter valve therapy (TVT) for patients with heart valve disease is one example of this ongoing data collection. Launched in 2012 and run by The Society of Thoracic Surgeons and the American College of Cardiology, the registry captures clinical information on patients in the United States undergoing transcatheter heart valve treatments. It has real-world patient data for more than 27,000 people and includes more than 300 data elements foreach participant. Collected information includes everything from patients’ self-reported quality of life to details on initial procedures and hospitalizations.
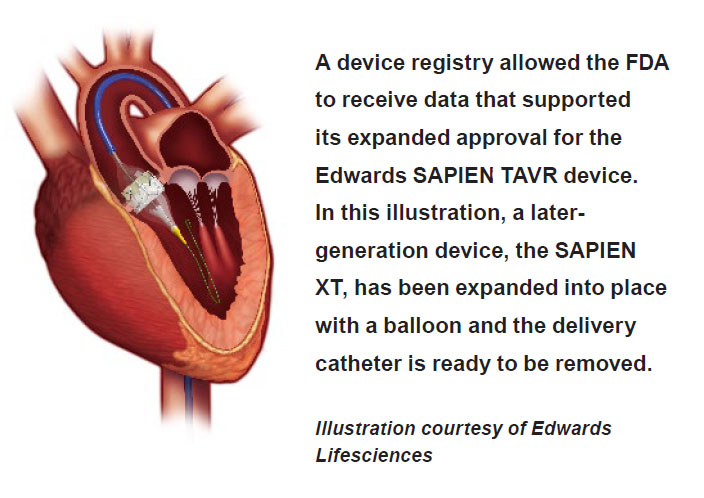
Background on the procedure and device: Transcatheter aortic valve replacement (TAVR) is a procedure that addresses heart valve abnormalities without the need for invasive, open-heart surgery. A collapsible replacement valve is delivered to the valve site via a catheter inserted through an artery. The replacement valve is then expanded, which pushes the old valve’s tissue flaps, called “leaflets,” out of the way.
The FDA first approved the Edwards SAPIEN TAVR device in 2011 to treat patients who, among other criteria, were not eligible for open heart surgery. The agency approved an expanded indication for the device less than a year later. But TAVR was still indicated only for high-risk patients who were candidates for surgery through the artery in the leg or via the apex of the heart (the lowest tip), excluding a significant number of patients who were poor candidates for these procedures.
In less than a year, data from the TVT Registry—in conjunction with data from FDA-approved clinical studies, and peer-reviewed medical journals—suggested that the Edwards SAPIEN device could offer good outcomes to patients needing other access sites, such as directly through the aorta. So in 2013, the FDA approved revisions to the device labeling to also cover inoperable and high-risk patients who need their devices inserted through alternative access points. The registry played a key role in this decision, as the FDA approved the labeling change based in large part on available registry data.
Today, the registry is required as a condition of approval on all TAVR therapies now available in the United States (Edwards SAPIEN Transcatheter Heart Valve, Edwards SAPIEN XT Transcatheter Heart Valve, and Medtronic CoreValve System).
Benefits of the registry:
- It confirms real-world data can be used to track and evaluate devices.
- It shows registries can help address postmarket questions and help regulators assess whether device approvals can be expanded to other patient groups.
- It continues to allow the FDA, clinical community, industry, payers and other stakeholders to follow the long-term performance of these devices using a rich repository of data, which can help patients.
The registry—just one of several ongoing FDA initiatives—is considered a model for successful collaboration among the FDA, professional societies, the Centers for Medicare & Medicaid Services (a payer), and industry. It will continue to serve as a national resource for real-world evidence for currently marketed devices and those submitted for future FDA review. In fact, Jeffrey E. Shuren, M.D., J.D., director of the Center for Devices and Radiological Health at the FDA, often cites the registry as an example of how the agency has efficiently met its goal to obtain an appropriate balance between data collected in the premarket setting and those collected postmarket.