FDA Facts: Biomarkers and Surrogate Endpoints
In the United States, people rely on the U.S. Food and Drug Administration to ensure that the drugs, biologics, or devices they use have acceptable risks and will help them feel or function better, or live longer. Clinical trials are conducted in order to demonstrate that new medical products deliver this positive balance of benefit and risk.
What are biomarkers?
Biomarkers, in layman’s terms, are defined characteristics that are measured as indicators of health, disease, or a response to an exposure or intervention, including therapeutic interventions (http://www.ncbi.nlm.nih.gov/books/NBK326791/). Biomarkers can help diagnose a disease, or predict future disease severity or outcomes, like measurements of blood pressure as an indicator of cardiovascular risks or measurements of blood sugar in diabetes. Biomarkers also are used to identify the best treatment for a patient, to monitor the safety of a therapy, or to find out if a treatment is having the desired effect on the body.
Many biomarkers used today have been developed to be used in a specific disease or as part of the development program for a specific medical product. Under the FDA’s recently established Biomarkers Qualification Program, biomarkers shown to be useful indicators across different development programs may be designated by the FDA as qualified biomarkers.
What are endpoints when used in a clinical trial?
A clinical trial’s “endpoints” are measurements of what happens to people in the trial. When a trial is intended to evaluate the efficacy and safety of a new medical product or a new use of an approved product, its endpoints usually measure benefit. Investigators typically use either clinical or surrogate endpoints.
Clinical outcomes are the most reliable clinical trial endpoints. They directly measure what matters most to people—whether they feel or function better, or live longer. Therapies can be recommended with confidence when clinical trials show that benefits, as measured by clinical outcomes, outweigh the adverse effects.
Surrogate endpoints are used instead of clinical outcomes in some clinical trials. Surrogate endpoints are used when the clinical outcomes might take a very long time to study, or in cases where the clinical benefit of improving the surrogate endpoint, such as controlling blood pressure, is well understood. Clinical trials are needed to show that surrogate endpoints can be relied upon to predict, or correlate with, clinical benefit. Surrogate endpoints that have undergone this testing are called validated surrogate endpoints and these are accepted by the FDA as evidence of benefit. Between 2010 and 2012, the FDA approved 45 percent of new drugs on the basis of a surrogate endpoint.
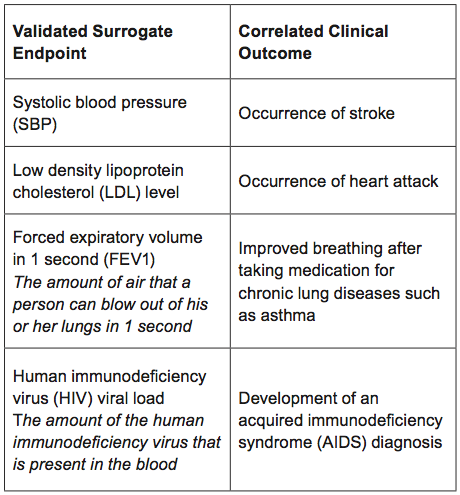
What are examples of validated surrogate endpoints used in medical product development?
There are many examples of validated surrogate endpoints used in specific settings, including those below.
Importantly, even validated surrogate endpoints can give misleading information about the overall risk and benefits of a medical product. Some therapies that have demonstrated improvement using a validated surrogate endpoint in one setting have been shown to be ineffective or even harmful in another, usually because of additional effects that are not measured by the surrogate.
Are surrogate endpoints that have not been validated useful in medical product approvals?
Reasonably likely surrogate endpoints may stand in for clinical outcomes, but are not yet validated. This is accomplished under FDA’s Accelerated Approval program, which is intended to provide patients with serious diseases more rapid access to promising therapies. Because reasonably likely surrogate endpoints used in the Accelerated Approval program have not been validated, sponsors must verify the predicted clinical benefit of their products with post-approval clinical trials.
Why are surrogate endpoints important for medical product development?
When a surrogate endpoint is clearly shown to predict a beneficial effect through appropriate studies, its use generally allows clinical studies to be conducted in smaller numbers of people over shorter periods of time. For example, a sufficient number of clinical trials have demonstrated that reducing systolic blood pressure reduced the risk of stroke. Hence, measurement of reduction in the surrogate endpoint of systolic blood pressure can stand in for the clinical outcome of stroke,and clinical trials targeting the reduction of risk of stroke can be conducted more rapidly in smaller populations using this validated surrogate endpoint.
What is the challenge of using biomarkers and surrogate endpoints in medical product development?
Biomarkers and surrogate endpoints alone do not give us the total picture of benefit and risk of a therapy. For example, surrogate endpoints may sometimes fail to predict the overall benefit and/or risk for a medical product. These limitations underscore the importance of continued evaluation in the post-market phase when products are approved based upon surrogate endpoints that have not been validated, as well as the need to rigorously evaluate and sometimes re-evaluate surrogate endpoints clinically. Despite such caveats, as science and technology advance, it is hoped that the increased use of biomarkers and surrogate endpoints will facilitate the more efficient development of safe and effective medical products.