COMPANY ANNOUNCEMENT
Busse Hospital Disposables Issues Nationwide Recall of Kits/Trays Containing Sterile Water Based Products Under Recall by Nurse Assist
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Medical Devices
- Reason for Announcement:
-
Recall Reason DescriptionDevice & Drug Safety - Potential Lack of Sterility
- Company Name:
- Busse Hospital Disposals
- Brand Name:
-
Brand Name(s)Busse Hospital Disposals
- Product Description:
-
Product DescriptionMedical Device Kits and Trays
Company Announcement
On November 21, 2023, Busse Hospital Disposals initiated a nationwide recall of medical device kits and trays containing (1) Nurse Assist part 6240, Stericare 100ML Sterile Saline Botle. This is the only saline contained in the trays/kits. Busse initiated this recall in direct response to Nurse Assist LLC’s, November 6, 2023, recall of 0.9% sodium chloride irrigation USP and sterile water for irrigation USP, due to potential lack of sterility assurance, which could result in a nonsterile product. In populations most at risk, such as patients who are immunocompromised, there is a possibility that the use of the affected product could potentially result in severe or life-threatening adverse events.
All distributors who are affected have been identified and notified through recall letters. The devices that have been shipped to your facility and identified as affected may be destroyed following instructions on recall letter. The recalled Busse kits/trays were manufactured and distributed to medical device distributors from July 2022 to November 2023.
The following products, which contain Nurse Assist Part#6240, Stericare 100ML Sterile Saline Botle have been recalled:
| Catalog Number | Product Descripon | Lot Number |
|---|---|---|
| 800 | Tracheostomy Care Tray – Sterile – Latex Free UDI: (01)00849233010000 |
2210099 |
| 2210135 | ||
| 2210337 | ||
| 2210377 | ||
| 2310181 | ||
| 2310233 | ||
| 2310291 | ||
| 1443 | Dressing Change Tray – Sterile – Latex Free UDI: (01)00849233010727 |
2210077 |
| 2210112 | ||
| 2310275 | ||
| 2310331 | ||
| 9251R1 | Tracheostomy Care Set – Sterile – Latex Free UDI: (01)00849233011069 |
2210087 |
| 2210165 | ||
| 2310361 |
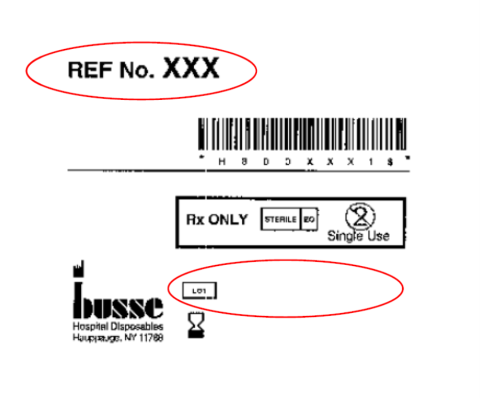
Products can be identified by locating the information above on their respective labeling – see reference below.
To date, Busse has not received any reports of serious injuries or deaths associated with this recall. Busse has notified both the FDA and affected distributors of the recall through direct communications. We remain commited to ensuring the safety and well-being of our customers.
Customers with any questions may contact Busse Hospital Disposables via telephone at 800-645-6526 Monday through Friday between the hours of 8am and 4pm (est) or via email at NurseAssistRecall@busseinc.com.
Customers may also contact Nurse Assist, LLC via telephone at 800-649-6800 Monday through Friday between the hours of 8am and 430pm (cst) or via e-mail at productremovalinfo@nurseassist.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
Company Contact Information
- Consumers:
- Busse Hospital Disposables
- 800-645-6526
- NurseAssistRecall@busseinc.com