Enforcement Report Information and Definitions
Important Note: The FDA Enforcement Report includes all recalls monitored by FDA to include Class I, II, III, or “not yet classified” as described in 21 CFR 7.50. It is important to note that most of the time recall classifications are determined after the recalling firm provides all of the information needed to determine the risk posed to public health. The classification and posting in the FDA Enforcement Report should not be seen as an expansion or change to a firm’s voluntary public warning. Firms often initiate voluntary recalls and provide public statements or notifications as part of their commitment to protecting consumers, which may occur well before the FDA completes its classification process and subsequently posts to this report.
How do I find recall information in the Enforcement Report?
FDA recall information is available on the Enforcement Report site by selecting the "View Weekly Enforcement Reports" button, the "Search Enforcement Reports" (i.e., Advanced Search) button, or by choosing the Archived Enforcement Reports link.
Alternatively, the Enforcement Report Application Programming Interface (API) allows access to data found in the Enforcement Report without the need to use the user interface. Information on how to gain access and how to use the API can be found at the Enforcement Report API Usage Documentation.
How do I get a weekly Enforcement Report?
Within the weekly Enforcement Report section, Users may access weekly reports by first selecting the desired year and month on the pull down menus and clicking on the links that correspond to the report week of interest.
How do I get a tailored Enforcement Report?
Users can also synthesize tailored reports via the "Search Enforcement Reports" (i.e., Advanced Search) section by selecting specific or a combination of criteria that includes:
- Product Description
- Code Information
- Product Type
- Recall Class
- Recalling Firm
- Status
- Recall Number
- Reason for Recall
- Classification Date
- Event ID
How do I search for a recall that has not been classified yet?
To search for products determined to meet the definition of a recall but have yet to be classified, select "Not Yet Classified" from the "Recall Class" drop-down menu. Selecting this will render the "Classified" date fields unusable; the reason being records identified as "Not Yet Classified" lack a classification date. All other fields will remain available for filtering the search results.
What are the different views for recall information?
Upon accessing the desired weekly or tailored report, users have four different ways to view recall information:
- Product View
- Event View
- Print View (only available under the weekly reports section)
- Export to CSV
Product View
The default view for the Enforcement Report is the Product View. This view displays recall information by product. To retrieve additional information about the recalled product, select the "VIEW DETAILS" link. A recall event may include more than one recalled product.
Event View
An alternate view of the Enforcement Report recall information will be the Event View. To choose this view, select the "View by Event" link to the right of the "View by Product" displayed under the page title. To retrieve additional information about a recall event, select the "VIEW DETAILS" link.
Print View
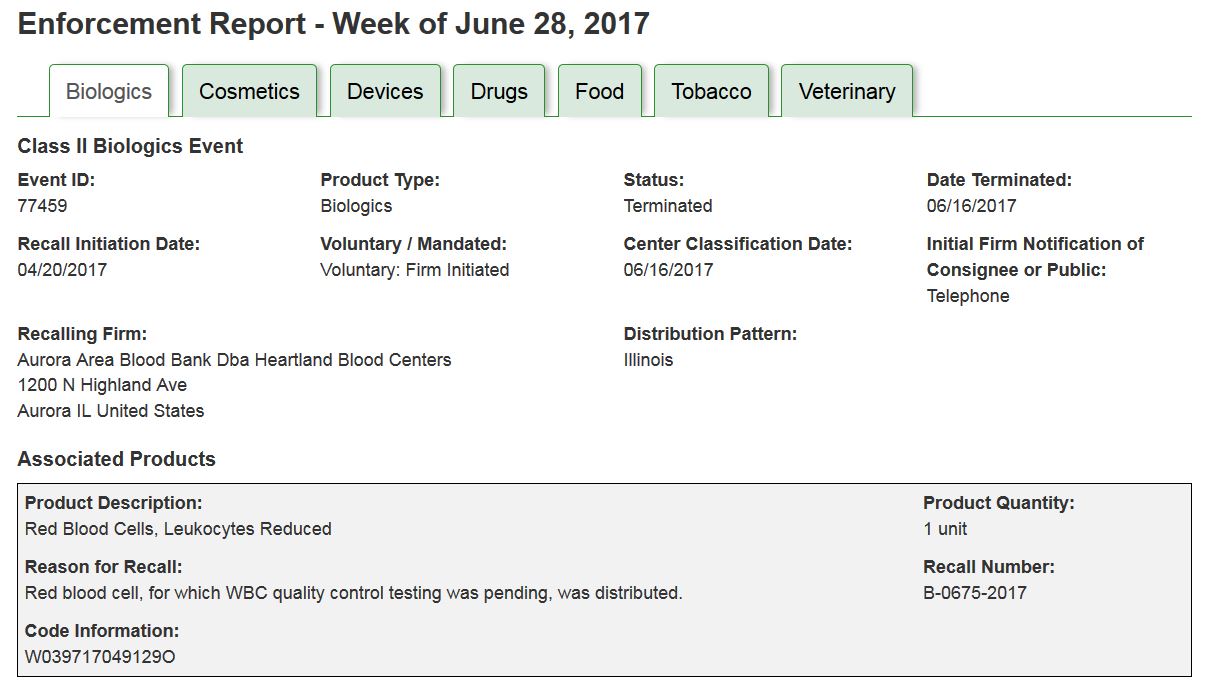
The ”Print View” (FIGURE 1) displays all the recall information for each Product Type, separated into tabs by commodity, on one page allowing users to more easily cut and paste information relevant to their needs.
FIGURE 1: Sample PRINT VIEW
Export to CSV
The “Export to CSV” view displays the search results into a CSV file in a table structure in plain text.
What are the report label definitions?
| Recalling Firm | The firm that initiates a recall |
| Classification | Numerical designation (I, II, or III) that is assigned by FDA to a particular product recall that indicates the relative degree of health hazard. For recalls pending classification, the entry will display as “Not Yet Classified” |
| Class I | Class I is a situation in which there is a reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death |
| Class II | Class II is a situation in which use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote |
| Class III | Class III is a situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences |
| Status | Shows the progress of a recall |
| On-Going | A recall which is currently in progress |
| Completed | A recall which has reached the point at which the firm has actually retrieved and impounded all outstanding product that could reasonably be expected to be recovered, or has completed all product corrections |
| Terminated | A recall will be terminated when the FDA determines that all reasonable efforts have been made to remove or correct the product in accordance with the recall strategy, and when it is reasonable to assume that the product subject to the recall has been removed and proper disposition or correction has been made commensurate with the degree of hazard of the recalled product |
| Distribution Pattern | General area of initial distribution such as states, countries, or territories. Note that subsequent distribution by the consignees to other parties may not be included |
| Product Description | Brief description of the product |
| Code Information | A list of all lot and/or serial numbers, product numbers, expiration dates, sell or use by dates, etc., which appear on the product or its labeling |
| Reason for Recall | Information describing how the product is defective |
| Product Quantity | The amount of product subject to recall |
| Voluntary/Mandated | Designates that a recall was initiated voluntarily by a firm on its own volition or after being requested to recall by FDA. “Mandatory” designates that a recall was initiated under a mandatory (statutory) recall authority, a court order, or FDA order. |
| Recall Initiation Date | The date that the firm first began notifying the public or their consignees of the recall |
| Initial Firm Notification of Consignee or Public | The method(s) by which the firm initially notified the public or their consignees of a recall |
| Recall Number | An alphanumeric designation assigned by FDA to a specific, classified recalled product (used for tracking purposes) |
| Event ID | A numerical designation assigned by FDA to a specific recall event (used for tracking purposes) |
| Center Classification Date | The date that FDA classified the recalled products as Class I, II, or III |
| Date Terminated | The date that FDA terminated the recall |
| Press Release URL(s) | The link(s) appearing in Press Release URL(s) section display the date the FDA published the press release and give direct access to the URL of the press release. If there is more than one press release for a recall, all the press releases will be listed. |