COMPANY ANNOUNCEMENT
Plastikon Healthcare Issues Voluntary Nationwide Recall of Milk of Magnesia Oral Suspension and Magnesium Hydroxide /Aluminum Hydroxide /Simethicone Oral Suspension Due to Microbial Contamination
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionDue to microbial contamination
- Company Name:
- Plastikon Healthcare, LLC
- Brand Name:
-
Brand Name(s)Major
- Product Description:
-
Product DescriptionMilk of Magnesia, Magnisium Hydroxide/Aluminum Hydroxide/Simethicone Oral Suspension
Company Announcement
FOR IMMEDIATE RELEASE – 6/07/2022 – Lawrence, Kansas – Plastikon Healthcare, LLC is voluntarily recalling one (1) lot of Milk of Magnesia 2400 mg/10 mL Oral Suspension, one (1) lot of Milk of Magnesia 2400 mg/30 mL Oral Suspension, eleven (11) lots of Magnesium Hydroxide 1200 mg/Aluminum Hydroxide 1200 mg/Simethicone 120 mg per 30 mL Oral Suspension, and two (2) lots of Magnesium Hydroxide 2400 mg/Aluminum Hydroxide 2400 mg/Simethicone 240 mg per 30 mL Oral Suspension to the consumer level. The products are being recalled due to microbial contamination.
Risk Statement: Administration or use of oral drug products with microbial contamination could potentially result in increased infections that may require medical intervention. Patients with compromised immune systems, such as patients in hospitals and nursing homes, have a higher probability of developing potentially life-threatening infections after taking a contaminated product. To date, Plastikon Healthcare has not received any reports of adverse events or injuries related to this recall.
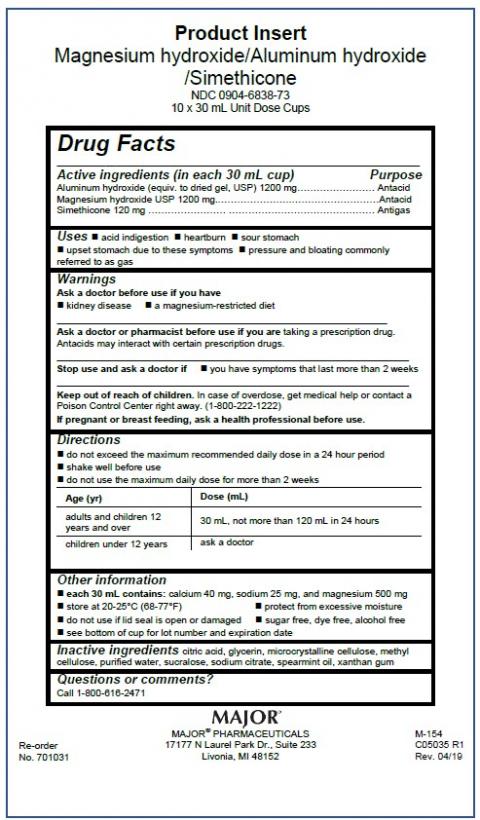
Product indication, lot numbers, expiration dates, and NDC information are listed in the table below. The products are packaged for institutional use and are sold to clinics and hospitals nationwide in single use cups with a foil lid. The affected lots were distributed to Major Pharmaceuticals Distribution Center (wholesaler) between 7/1/2020 and 10/31/2021, who shipped to hospitals, nursing homes, and clinics nationwide. The products are private labeled for Major Pharmaceuticals.
| Product Name | Milk of Magnesia 2400 mg / 30 mL Oral Suspension | Milk of Magnesia 2400 mg / 10 mL Oral Suspension | Magnesium Hydroxide 1200 mg / Aluminum Hydroxide 1200 mg / Simethicone 120 mg per 30 mL Oral Suspension | Magnesium Hydroxide 2400 mg / Aluminum Hydroxide 2400 mg / Simethicone 240 mg per 30 mL Oral Suspension |
| Indications for Use | Occasional relief of constipation (irregularity) in adults and children 12 years and older or for children under 12 as recommended by a doctor. | Occasional relief of constipation (irregularity) in adults and children 12 years and older or for children under 12 as recommended by a doctor. | Relief of acid indigestion, heartburn, sour stomach, upset stomach due to these symptoms, pressure and bloating commonly referred to as gas. | Relief of acid indigestion, heartburn, sour stomach, upset stomach due to these symptoms, pressure and bloating commonly referred to as gas. |
| Lot/Exp. | 20071A / Jul. 2022 | 20074A / Jul. 2022 | 21103A / Sep. 2023 20046A / May. 2022 20079A / Aug. 2022 20080A / Aug. 2022 20081A / Aug. 2022 21057A / May. 2023 21059A / May. 2023 21095A / Sep. 2023 21096A / Sep. 2023 21099A / Sep. 2023 21115A / Oct. 2022 |
20051A / Aug. 2022 20088A / Sep. 2022 |
| NDC | 0904-6846-73 | 0904-6840-72 | 0904-6838-73 | 0904-6839-73 |
| Packaging | Carton containing 100 single dose cups (10 trays x 10 cups) | Carton containing 100 single dose cups (10 trays x 10 cups) | Carton containing 100 single dose cups (10 trays x 10 cups) | Carton containing 100 single dose cups (10 trays x 10 cups) |
| Product Identification | See image below | See image below | See image below | See image below |
Plastikon Healthcare is notifying its direct customers via a recall letter to arrange for return of any recalled product. Anyone with an existing inventory of the lots which are being recalled should stop use and distribution, and quarantine immediately. Return all quarantined product to the place of purchase. For clinics, hospitals, or healthcare providers that have dispensed product to patients, please notify patients regarding the recall.
Consumers with questions regarding this recall can contact Plastikon Healthcare by phone at (785) 330-7109 or by e-mail at sdixon@plastikon.com Monday through Friday from 9 am to 4 pm CST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Link to Expanded Recall