COMPANY ANNOUNCEMENT
Meitheal Pharmaceuticals, Inc. Issues Voluntary Nationwide Recall of Cisatracurium Besylate Injection, USP 10mg per 5mL Due to Mislabeling
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionMislabeling
- Company Name:
- Meitheal Pharmaceuticals, Inc.
- Brand Name:
-
Brand Name(s)Meitheal Pharmaceuticals, Inc.
- Product Description:
-
Product DescriptionCisatracurium Besylate Injection, USP 10mg per 5mL
Company Announcement
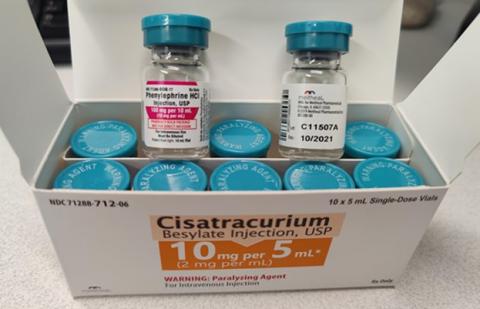
Meitheal Pharmaceuticals, Inc. (“Meitheal”), announced today that it is voluntarily recalling one (1) lot of Cisatracurium Besylate Injection, USP 10mg per 5mL to the user level. The decision to recall the product was made after a product complaint revealed that a portion of Lot C11507A of cartons labeled as Cisatracurium Besylate Injection, USP 10mg per 5mL, containing 10-vials per carton, contained 10-vials mis-labeled as Phenylephrine Hydrochloride Injection, USP 100mg per 10mL. To date, Meitheal has not received reports of any adverse events or identifiable safety concerns attributed to the lot.
There is a reasonable probability that a patient who requires cisatracurium for muscle paralysis as part of general anesthesia is administered phenylephrine instead would not receive any skeletal muscle relaxation and could cause a hyperadrenergic state resolution in elevated blood pressure, arrhythmia and cardiac/brain ischemia. If this is not quickly diagnosed and treated, severe illness or death can occur.
There is a reasonable probability that a patient who requires phenylephrine to increase their blood pressure, such as patients with severely low blood pressure, especially resulting from septic shock who is administered cisatracurium instead could result in a fast onset of muscle paralysis and decrease in oxygen. If this is not quickly diagnosed and treated, severe illness or death can occur within minutes.
Cisatracurium Besylate Injection is used as a nondepolarizing neuromuscular blocker. The affected Cisatracurium Besylate Injection lot being recalled is C11507A, EXP. October 2021. The product can be identified as a 5mL vial stoppered with a rubber stopper and sealed with aluminum seal having an Aqua color flip-off seal. Meitheal commenced shipping the product to customers on August 19, 2020 which was distributed to wholesalers nationwide in the USA.
| Product | Lot Number | Expiration Date | NDC Number | Distribution Dates |
|---|---|---|---|---|
| Cisatracurium Besylate Injection, USP 10mg per 5mL |
C11507A*
|
October 2021*
|
71288-712-06 (unit of sale) 71288-712-05 (unit of use) |
August 19, 2020 – January 04, 2021 |
| *Note: Mis-labeled product will have this same Lot Number of C11507A and Expiration Date of October 2021 but will be labeled on the vial as Phenylephrine Hydrochloride Injection, USP 100mg per 10mL, NDC 71288-808-77 (unit of use). | ||||
Meitheal has notified its distributors and customers in writing to arrange for return of all recalled product. Customers who have purchased the product should not open the carton or use its contents and should immediately quarantine and return the recalled lot of product. Customers who may have further distributed this product should promptly identify their customers and notify them at once of this product recall.
Consumers with questions regarding this recall can contact Meitheal’s Customer Service weekdays 8:00AM to 6:00PM CST at 844-824-8426. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax, as follows.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
ABOUT MEITHEAL PHARMACEUTICALS
Since 2017, Meitheal Pharmaceuticals has bridged critical gaps in the US healthcare market by supplying high quality, affordable generic injectables. Our diversified product range — from antibiotics, anticoagulants, and muscle relaxants to drugs used in chemotherapy — represents practical solutions for countless patients around the country, as well as Meitheal’s commitment to their care. Based in Chicago, Illinois, our aim each day is producing quality and ensuring affordability, using the traditional Irish guiding principle we are named for — Meitheal (Mee·hall): working together toward a common goal, for the greater good.
Learn more about who we are and what we do at www.meithealpharma.com.
Company Contact Information
- Consumers:
- Meitheal’s Customer Service
- 844-824-8426
- More information
-
Official URLwww.meithealpharma.com
- Media:
- Camilla White
- 646-250-0050
- camilla.white@fticonsulting.com