COMPANY ANNOUNCEMENT
Hospira Issues A Voluntary Nationwide Recall for One Lot of Sterile Water for Injection, USP, Due to the Potential Presence of Visible Particulate
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionDue to visible particulate
- Company Name:
- Hospira, Inc.
- Brand Name:
-
Brand Name(s)Hospira, Inc.
- Product Description:
-
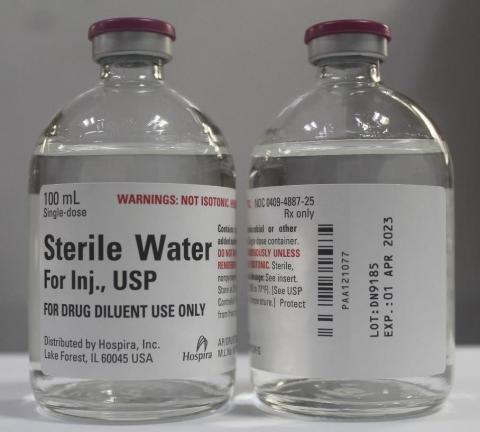
Product DescriptionSterile Water for Injection, USP, 100 mL Single Dose Glass Fliptop Vial
Company Announcement
Hospira, Inc., a Pfizer company, is voluntarily recalling lot DN9185 of Sterile Water for Injection, USP, 100 mL Single Dose Glass Fliptop Vial, to the hospital/institution level due to a confirmed customer report for a single vial with a visible particulate.
Hospira’s assessment of the potential risk to patients concluded that in rare instances the use of the impacted product can be associated with potential adverse events such as anaphylaxis, fever, gastrointestinal disturbances, vein irritation, localized vein inflammation, phlebitis, end-organ granuloma, tissue ischemia, pulmonary emboli, and infarction. To date, Hospira, Inc. has not received reports of any adverse events associated with this issue for this lot.
Sterile Water for Injection USP is a sterile, nonpyrogenic preparation of water for injection which contains no bacteriostat, antimicrobial agent or added buffer and is supplied only in single-dose containers. This parenteral preparation is indicated only for diluting or dissolving drugs for intravenous,
intramuscular or subcutaneous injection, according to instructions of the manufacturer of the drug to be administered. The NDC, Lot Number, Expiration Date, and Configuration details for Sterile Water for Injection, USP, is indicated in the table below and a photo of the product can be found at the end of this press release. The product lot was distributed nationwide to wholesalers/distributors/and hospitals in the United States from October to December 2020.
| Product | NDC | Lot Number | Expiration Date | Presentation | Configuration /Count |
|---|---|---|---|---|---|
| Sterile Water for Injection, USP 100 mLbr Single-dose Glass Fliptop Vial |
Vial: 0409-4887-25 Carton: 0409-4887-99 |
DN9185 | 01 Apr 2023 | 100 mL, Single-dose Glass Fliptop Vial | Tray of 25 vials |
Hospira, Inc., places the utmost emphasis on patient safety and product quality at every step in the manufacturing and supply chain process. Hospira, Inc. has notified wholesalers/ distributors/hospitals by letter to arrange for return of any recalled product.
Wholesalers, distributors or hospitals with an existing inventory of the lot, which is being recalled, should stop use and distribution and quarantine immediately. If you have further distributed the recalled product, to the wholesale or hospital level/institution, please notify any accounts or additional locations which may have received the recalled product from you. Hospitals/Institutions should inform Healthcare Professionals in your organization of this recall. For additional assistance, call Stericycle at 1-800-805-3093 between the hours of 8 a.m. to 5 p.m. ET, Monday through Friday.
Healthcare Professionals with questions regarding this recall can contact Pfizer using the below information.
| Contact Center | Contact Information | Area of Support |
|---|---|---|
| Pfizer Medical Information | 800-438-1985, option 3 |
For medical questions regarding the product |
| Pfizer Drug Safety | 800-438-1985, option 1 (24 hours a day; 7 days a week) |
To report adverse events and product complaints |
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Stericycle
- 1-800-805-3093
- Media:
- Eamonn Nolan
- 212-733-4626
- Eamonn.Nolan@pfizer.com