FY2015 Regulatory Science Research Report: Pharmacokinetic/Pharmacodynamic Models and Pharmacometrics
Introduction
The success of model-based drug development has generated strong public interest in applying modeling and simulation (M&S) tools for the evaluation of therapeutic equivalence between generic drugs and their reference products. In the past few years, OGD has increasingly used PK/PD M&S approaches to assist regulatory decision making in BE study design, BE metrics, and clinically relevant criteria. These approaches are especially valuable for products that have non-traditional PK behavior or present specific safety concerns. These products include, but are not limited to, locally-acting drugs, complex nano formulations, MR oral solid products, long-acting parenteral products, and NTI drugs. Despite the large number of new drug application (NDA) submissions that include M&S support, industry’s use of M&S support for ANDA submissions is limited. In part, this is because M&S in the generic drug framework has not received full recognition; communication between industry and FDA has been lacking in this regard; and gaps in knowledge exist pertaining to within-subject variability of physiological parameters and the interaction between formulation variables and physiological factors. M&S tools are essential to developing a modern ANDA review process, and to this end, we propose an innovative model for future ANDA development.
Research
DQMM promotes the use of pharmacometric tools for equivalence evaluation to facilitate knowledge-based regulatory decision making prior- and post-ANDA approval. M&S approaches were used in developing BE guidance and across all phases of FDA’s ANDA regulatory review. ORS is also collaborating with external experts by awarding scientific grants to develop innovative pharmacometric tools for equivalence evaluation. These efforts include, but are not limited to, identifying appropriate PK metric(s) in BE evaluation; classifying NTI drugs using systematic approaches; evaluating issues related to generic drug substitution by model and systems pharmacologic-based approaches; and simulating clinical BE trials.
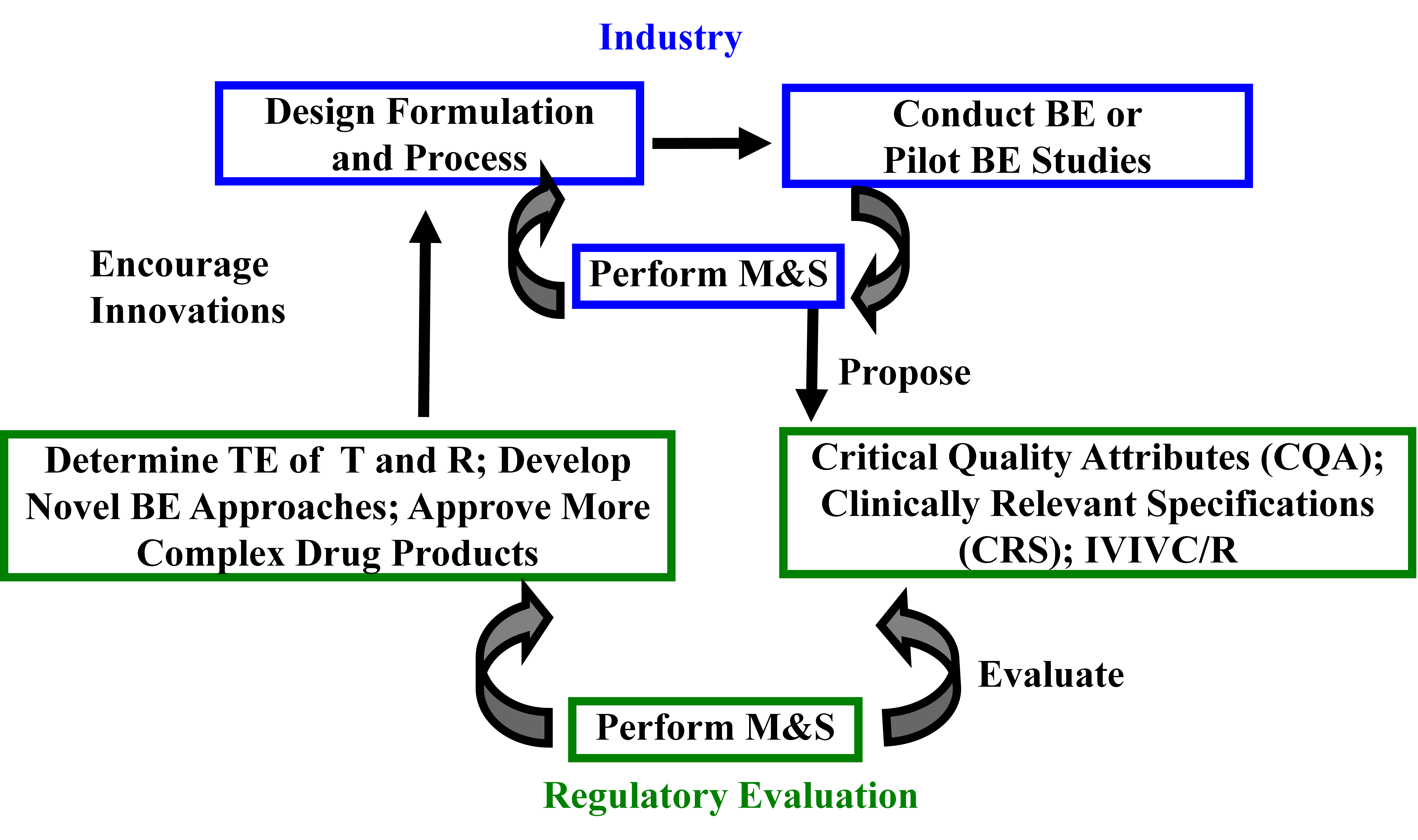
Figure 20. Innovative model for future ANDAs
Legend: R and T are reference and test products, respectively. QbD stands for quality by design.
Source: Robert Lionberger. Application of PBPK models in assessment of bioequivalence (AAPS Annual Meeting 2014)
ORS staff facilitating research in this area
- Lanyan Fang, Hyewon Kim, Nan Zheng, Andrew Babiskin, Edwin Chow, and other ORS members
Projects and Collaborators
- Population pharmacokinetic and pharmacodynamic, dose-toxicity modeling and simulation for narrow therapeutic index (NTI) drugs
- Site PI: Jogarao V Gobburu
- Grant #: 1U01FD005188-01
- Pharmacometric modeling and simulation for a generic drug substitutability evaluation and post marketing risk assessment
- Site PI: Jogarao V Gobburu
- Grant #: 1U01FD005192-01
- A model- and systems-based approach to efficacy and safety questions related to generic substitution
- Site PI: Lawrence Lesko
- Grant #: 1U01FD005210-01
- Pharmacometric modeling of immunosuppressants for evaluation of bioequivalence criteria
- Site PI: Catherine Mary Turner
- Grant #: 1U01FD005191-01
- Collection of dose adjustment and therapeutic monitoring data to aid narrow therapeutic index drug classification
- Site PI: Michael Cohen-Wolkowiez
- Grant #: 1U01FD004858-01
Publications and Presentations
- Babiskin A, Kim H, Fang L, Lapteva L, Jiang W, Lionberger R. Use of partial AUC to demonstrate bioequivalence of generic methylphenidate extended release products using physiological-based absorption modeling and simulation. American Society for Clinical Pharmacology and Therapeutics (ASCPT) Annual Meeting, New Orleans LA (Mar 3-7, 2015)
- Zheng et al. Modeling and simulation of bioequivalence between disparate generic antiepileptic drugs. AAPS (2012)
- Zheng et al. Modeling and simulation of iron transport after single dose of iron colloid injection. AAPS (2013)
- Lesko et al. Integrated data mining and systems pharmacology to explore the comparative safety of brand-name and generic drugs. Population Approach Group in Europe, Hersonissos, Crete, Greece (2015)
- Lesko et al. A model- and systems-based approach to efficacy and safety questions related to generic substitution. American College of Clinical Pharmacy (ACCP) , San Francisco CA (2015)
- Lesko et al. Use of physiologically based pharmacokinetic modeling as a computational and mathematical tool to evaluate the switchability of generic and brand name products. ACCP, San Francisco CA (2015)
Outcomes
- Research projects in progress