Real-world Evidence from a Narrow Therapeutic Index Product (Levothyroxine) Reflects the Therapeutic Equivalence of Generic Drug Products
Background
The National Institute of Diabetes and Digestive and Kidney Diseases reports that almost 5% of the U.S. population above the age of 12 years have hypothyroidism, a condition characterized by insufficient hormonal output by the thyroid gland. This prevalence of disease carries economic implications for the health care sector. Levothyroxine is the most commonly prescribed thyroid hormone medicine used to treat hypothyroidism and is one of the most widely prescribed drug products in the United States. Given this high prescription rate, the FDA approval of multiple generic products has provided patients with additional options for filling levothyroxine prescriptions and has reduced health care costs for millions of patients.1
Despite significant increases in generic prescription rates in the last decade, a recent study shows that some physicians, especially endocrinologists, tend to prescribe generic levothyroxine products less often than they prescribe brand-name levothyroxine products.2 The reasons for this tendency are not fully clear, but levothyroxine is a medicine with a narrow therapeutic index, meaning that relatively small deviations from the proper dose can cause a clinically meaningful shift in pharmacological effects when administered to a patient. It is possible that some levothyroxine prescribers, in favoring brand-name product out of concern over the narrow therapeutic index of the drug, are not aware that FDA bioequivalence standards ensure that generic products do not differ in safety, efficacy, or quality (including potency) from regulatory standards that govern brand-name products.3 Indeed, the FDA has recommended that approval of generic levothyroxine products be based on a detailed, complex bioequivalence study design with narrower margin for allowable pharmacokinetic differences between the generic and brand-name products.
CDER scientists and their colleagues at Yale University-Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI)4 recently investigated the clinical initiation of levothyroxine over a ten-year period (2008-2017) to address the residual concern from the clinical community as to whether generic and brand-name levothyroxine have proven equally effective for patients with mild hypothyroidism. This retrospective study was designed to verify the importance of stringent approval criteria and quality control specifications that the FDA applies to generic and brand-name products, including those with a narrow therapeutic index.
CDER Comparison of Real-World Outcomes Using Generic and Brand-name Levothyroxine
In order to assess the comparative effectiveness of generic versus brand levothyroxine in patients with mild hypothyroidism, CDER scientists, in collaboration with academic experts at Yale Univ. and Mayo Clinic, used a national administrative claims database to identify patients who first started levothyroxine between 2008 and 2017.5 The study examined the effect of generic versus brand-name levothyroxine use on the clinical management of thyroid status (as measured by levels of thyroid stimulating hormone), information that should be relevant to clinicians who prescribe levothyroxine. More generally, the findings may sustain the clinically and economically responsible management of millions of patients with hypothyroidism in the United States.
The researchers reviewed claims for 15,299 patients filling generic levothyroxine and 2,299 patients filling brand-name levothyroxine for the first time during the time period investigated, verifying that impaired thyroid status recorded for patients prior to treatment was ameliorated over the course of the established treatment protocol. These pools of patients allowed the researchers to characterize patients according to features such as demographics (e.g., age, sex, race) and accordingly establish thousands of matches suitable for comparing the response to generic or brand-name levothyroxine treatment. They found similar attainment of normal thyroid status following levothyroxine treatment for patients with mild or subclinical hypothyroidism, regardless of whether generic or brand-name medicine had been administered.
Implications
The approximate six-to-one ratio for generic-to-brand levothyroxine users is consistent with an increase in the prescribing of generic levothyroxine products observed in a recent study.2 However, the rate at which generic levothyroxine is prescribed lags the rate at which other generic drugs are prescribed. This lag may reflect the hesitancy of some prescribers to prescribe generic levothyroxine, but the real-world evidence provided from the study described here should assure clinicians and patients that generic levothyroxine is as effective as brand-name levothyroxine as initial therapy for hypothyroidism. CDER scientists are committed to generating real-world evidence for the use of generics, particularly for narrow therapeutic index products, and continue to pursue studies that examine clinical outcomes and the effects of switching between brand-name and generic levothyroxine for the treatment of hypothyroidism.
The Spotlight series presents generalized perspectives on ongoing research- and science-based activities within CDER. Spotlight articles should not be construed to represent FDA’s views or policies.
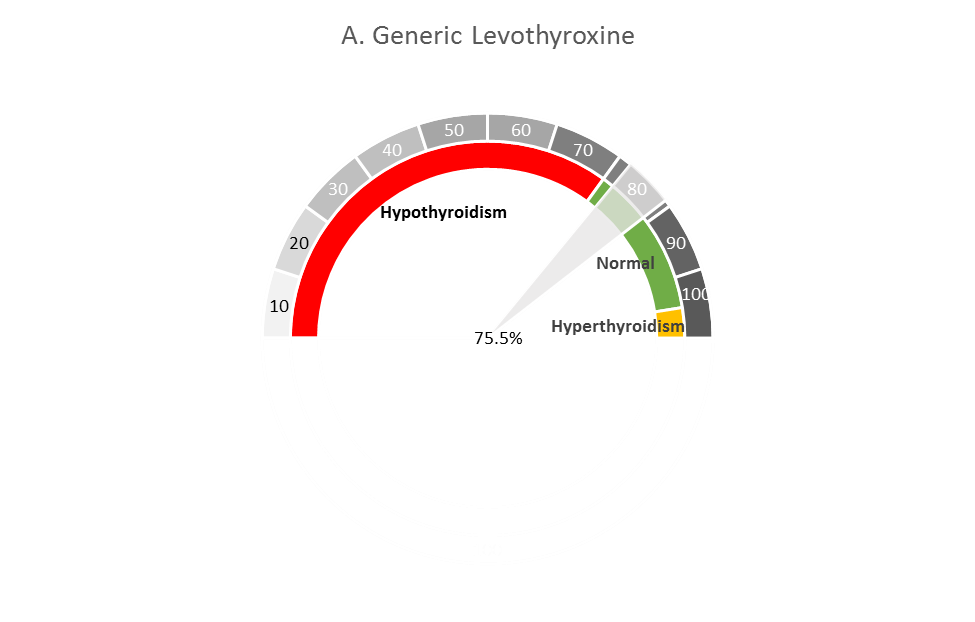
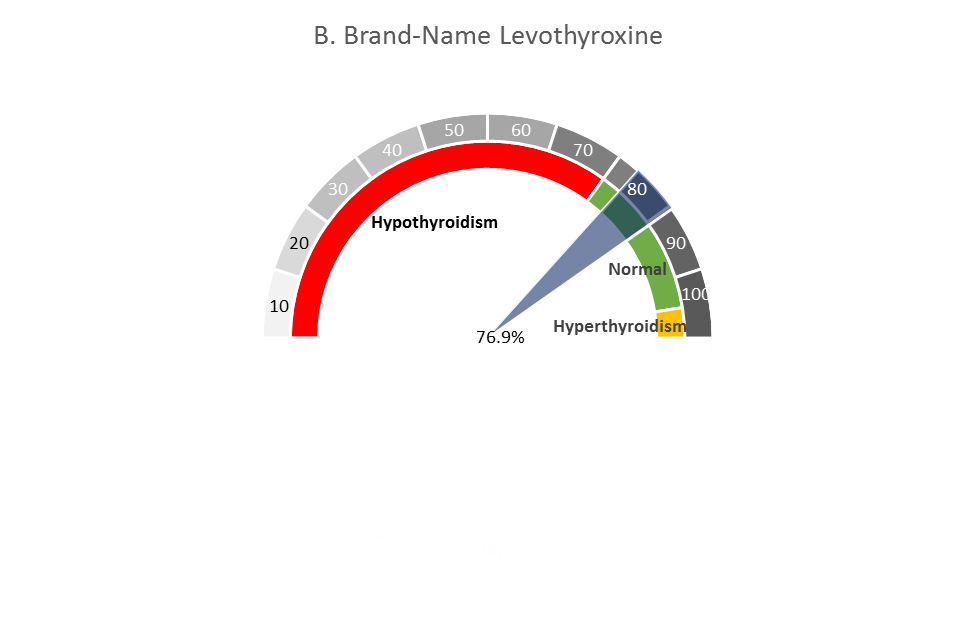
CDER scientists and their collaborators at Yale Univ. and Mayo Clinic have inspected administrative health claims from patients with mild hypothyroidism to compare generic vs. brand-name levothyroxine products in restoring normal levels of thyroid stimulating hormone (TSH) levels. In the images shown here, the spectrum of gray shades indicates lower levels of thyroid hormone (lighter gray, from left of spectrum) to higher levels of thyroid hormone (with abnormally high hormone levels at the far right). The percentages (75.5% in A, and 76.9% in B) indicate, respectively, that generic and brand-name levothyroxine products achieved comparable restoration of thyroid levels in patients. The lower and upper bounds (95% confidence interval) of these estimated proportions are shown by the edges of the wedge (gray in A, blue in B), with the average displayed at the center of each wedge.
1 Rodriguez-Gutierrez R, Maraka S, Ospina NS et al. Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol. 2017 Apr;5(4):246-248.
2 Ross JS, Rohde S, Sangaralingham L et al. Generic and brand-name thyroid hormone drug use among commercially insured and medicare beneficiaries, 2007 through 2016. J Clin Endocrinol Metab. 2019 Jun 1;104(6):2305-2314.
3 FDA Acts to Ensure Thyroid Drugs Don’t Lose Potency Before Expiration Date. https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm161259.htm. Accessed August 8, 2019.
4 This work was supported by a Center of Excellence in Regulatory Science and Innovation (CERSI) grant to Yale University and Mayo Clinic from the US Food & Drug Administration (U01FD005938). Scientists from CDER Office of Generic Drugs (OGD), Office of Surveillance and Epidemiology (OSE), and Office of Translational Sciences (OTS) jointly contributed to this work.
5 Brito JP, Ross JS, Sangaralingham L et al. Comparative effectiveness of generic vs brand-name levothyroxine in achieving normal thyrotropin levels. JAMA Netw Open. 2020 Sep 30;3(9):e2017645. doi:10.1001/jamanetworkopen.2020.17645.