FAERS Reporting by Patient Outcomes by Year
(As of November 2015)
Back to FAERS Statistics Main Page
These data describe the outcome of the patient as defined in U.S. reporting regulations (21 CFR 310.305, 314.80, 314.98, 600.80) and Forms FDA 3500 and 3500A (the MedWatch forms). Serious means that one or more of the following outcomes were documented in the report: death, hospitalization, life-threatening, disability, congenital anomaly and/or other serious outcome. Documenting one or more of these outcomes in a report does not necessarily mean that the suspect product(s) named in the report was the cause of these outcomes.
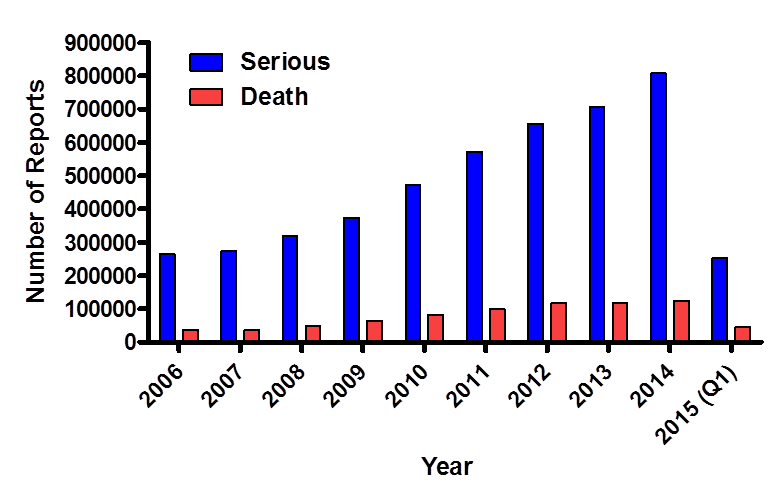
This figure illustrates the patient outcome(s) for reports in FAERS since the year 2006 until the first quarter of 2015. Serious outcomes include death, hospitalization, life-threatening, disability, congenital anomaly and/or other serious outcome.
Figure 4. This figure illustrates the patient outcome(s) for reports in FAERS since the year 2006 including reports until the first quarter of 2015. Serious outcomes include death, hospitalization, life-threatening, disability, congenital anomaly and/or other serious outcome.
| Year | Deaths | Serious |
|---|---|---|
| 2006 | 37,309 | 264,227 |
| 2007 | 36,689 | 272,324 |
| 2008 | 49,699 | 318,536 |
| 2009 | 63,830 | 373,471 |
| 2010 | 82,704 | 471,243 |
| 2011 | 98,469 | 572,992 |
| 2012 | 117,202 | 656,613 |
| 2013 | 116,388 | 707,593 |
| 2014 | 123,927 | 807,270 |
| 2015(Q1) | 44,693 | 253,017 |