Drug Trials Snapshots: RYZNEUTA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the RYZNEUTA Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
RYZNEUTA (efbemalenograstim alfa)
rīz-new-ta
Evive Biotechnology
Approval date: November 16, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
RYZNEUTA is a prescription drug that is a leukocyte growth factor indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in adult patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of febrile neutropenia.
How is this drug used?
ARYZNEUTA is a subcutaneous injection that is taken once approximately 24 hours after cytotoxic chemotherapy.
Who participated in the clinical trials?
The FDA approved RYZNEUTA based on evidence from two main clinical trials, GC-627-04 and GC-627-05, in 515 patients with breast cancer receiving chemotherapy. The trials were conducted at 52 sites in 5 countries including Hungary, Russia, Ukraine, Bulgaria, and the United States.
There was one patient included in the trial from the United States, and 514 patients were included from sites outside of the United States.
The same trials (GC-627-04 and GC-627-05) were used to assess efficacy and safety.
How were the trials designed?
RYZNEUTA was evaluated in two main clinical trials that were randomized and controlled. A total of 515 patients were randomized to receive RYZNEUTA or placebo, or Neulasta, after receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of febrile neutropenia to treat metastatic breast cancer. Both trials evaluated the benefit and side effects of RYZNEUTA in patients.
The benefit of RYZNEUTA was based on the mean duration of severe neutropenia seen in patients after receiving either RYZNEUTA or control (placebo or Neulasta).
How were the trials designed?
The safety and efficacy of RYZNEUTA were established in two main clinical trials, GC-627-04 and GC-627-05, in 515 patients with breast cancer receiving chemotherapy.
Trial GC-627-04 was a randomized, double-blind, placebo-controlled study that employed doxorubicin 60 mg/m2 and docetaxel 75 mg/m2 administered every 21 days for up to four cycles for the treatment of metastatic or nonmetastatic breast cancer. A total of 122 female patients were randomized to receive a single subcutaneous injection of RYZNEUTA 20 mg (N=83) or placebo (N=39) on Day 2 of chemotherapy cycle 1. All patients received RYZNEUTA (20 mg) on Day 2 of chemotherapy cycles 2 through 4.
Trial GC-627-05 was a randomized, active-controlled study that compared RYZNEUTA to Neulasta that employed docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 administered every 21 days for up to four cycles for the treatment of nonmetastatic breast cancer. A total of 393 female patients were randomized to receive a single subcutaneous injection of RYZNEUTA 20 mg (N=197) or Neulasta 6 mg (N=196) on Day 2 of each chemotherapy cycle.
The demonstration of the efficacy of RYZNEUTA for both trials was based on the mean days of severe neutropenia in cycle 1.
DEMOGRAPHICS SNAPSHOT:
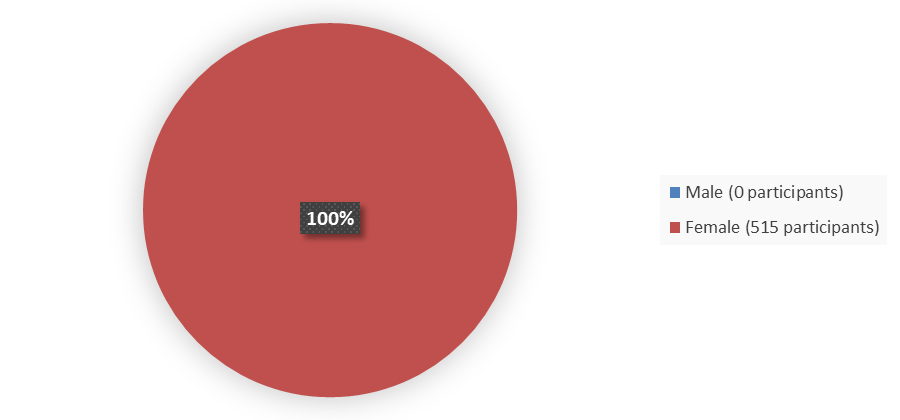
Figure 1 summarizes how many participants by sex were enrolled in the clinical trials used to evaluate the efficacy of RYZNEUTA.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
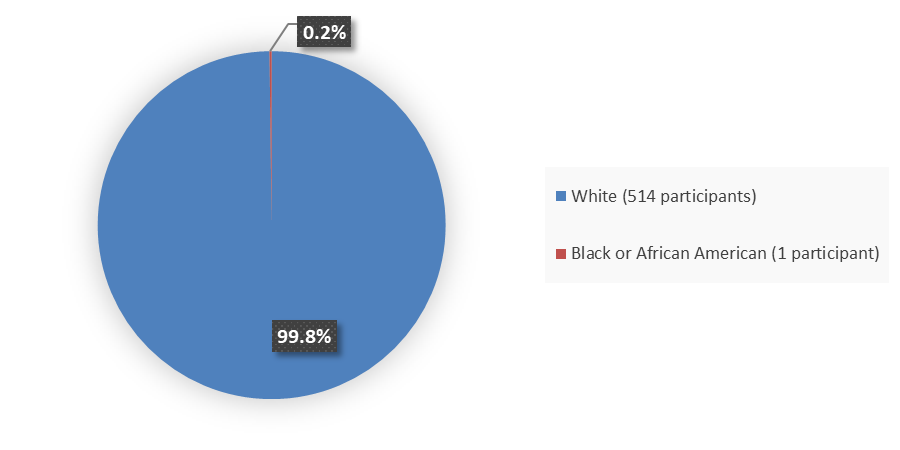
Figure 2 summarizes the percentage of participants by race enrolled in the clinical trials used to evaluate the efficacy of RYZNEUTA.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
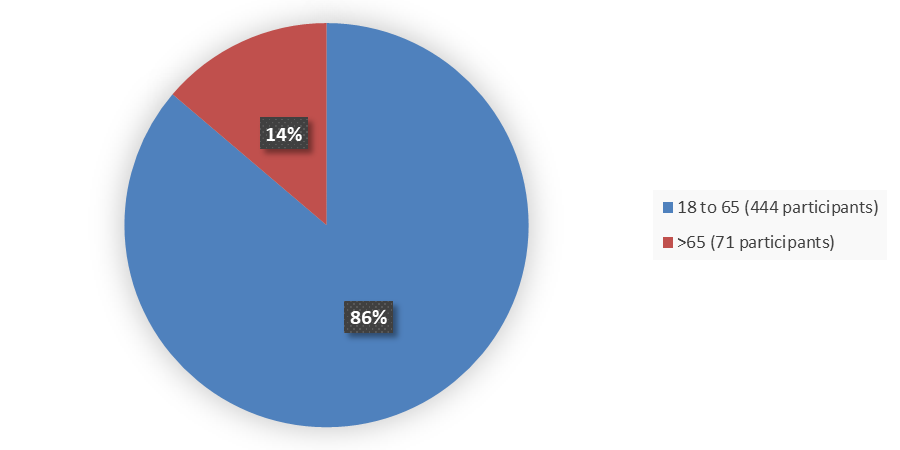
Figure 3 summarizes the percentage of participants by age enrolled in the clinical trials used to evaluate the efficacy of RYZNEUTA.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
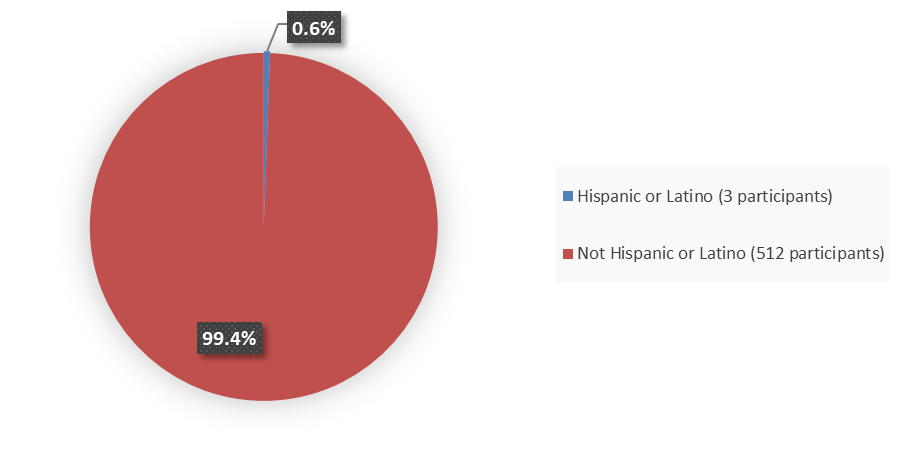
Figure 4 summarizes the percentage of participants by ethnicity enrolled in the clinical trials used to evaluate the efficacy of RYZNEUTA.
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Baseline Demographics of Efficacy Trials by Age, Race, Sex, and Ethnicity
| Characteristic |
GC-627-04 |
GC-627-05 |
Total RYZNEUTA N=280 |
||
|
RYZNEUTA |
Placebo |
RYZNEUTA |
Neulasta |
||
|
Sex, n (%) |
|
|
|
|
|
|
Female |
83 (100) |
39 (100) |
197 (100) |
196 (100) |
280 (100) |
|
Age, years |
|
|
|
|
|
|
Mean (SD) |
50.8 (9.3) |
51.5 (9) |
51.4 (11.8) |
53.4 (11.1) |
51.2 (11.1) |
|
Median (min, max) |
50 (30, 69) |
51 (33, 67) |
53 (28, 83) |
55 (26, 80) |
52 (28, 83) |
|
Age group, years, n (%) |
|
|
|
|
|
|
<65 |
76 (91.6) |
36 (92.3) |
171 (86.8) |
161 (82.1) |
247 (88.2) |
|
≥65 |
7 (8.4) |
3 (7.7) |
26 (13.2) |
35 (17.9) |
33 (11.8) |
|
Age group ≥75, years, n (%) |
|
|
|
|
|
|
≥75 years |
0 |
0 |
3 (1.5) |
1 (0.5) |
3 (1.1) |
|
Race, n (%) |
|
|
|
|
|
|
Black or African American |
1 (1.2) |
0 |
0 |
0 |
1 (0.4) |
|
White |
82 (98.8) |
39 (100) |
197 (100) |
196 (100) |
279 (99.6) |
|
Ethnicity, n (%) |
|
|
|
|
|
|
Hispanic or Latino |
1 (1.2) |
1 (2.6) |
0 |
1 (0.5) |
1 (0.4) |
|
Hispanic or Latino |
82 (98.8) |
38 (97.4) |
197 (100) |
195 (99.5) |
279 (99.6) |
|
Country of participation, n (%) |
|
|
|
|
|
|
Bulgaria |
0 |
0 |
18 (9.1) |
18 (9.2) |
18 (6.4) |
|
Hungary |
4 (4.8) |
1 (2.6) |
23 (11.7) |
22 (11.2) |
27 (9.6) |
|
Russia |
32 (38.6) |
16 (41.0) |
73 (37.1) |
72 (36.7) |
105 (37.5) |
|
Ukraine |
46 (55.4) |
22 (56.4) |
82 (41.6) |
84 (42.9) |
128 (45.7) |
|
United States |
1 (1.2) |
0 |
1 (0.5) |
0 |
2 (0.7) |
Source: Adapted from FDA Review
What are the benefits of this drug?
The studies demonstrated that the mean duration of severe neutropenia in cycle 1 was lower for RYZNEUTA-treated patients as compared to placebo-treated patients, and the mean days of severe neutropenia of RYZNEUTA-treated patients did not exceed that of Neulasta-treated patients by more than 0.6 days (prespecified noninferiority margin) in cycle 1 of chemotherapy. RYZNEUTA was noninferior to Neulasta and more effective than placebo at decreasing the duration of severe neutropenia, which in patients receiving myelosuppressive chemotherapy can reduce complications associated with cancer treatment including hospitalization and life-threatening infection.
What are the benefits of this drug (results of trials used to assess efficacy)?
Study GC-627-04 was a randomized, double-blind, placebo-controlled study in which patients received the chemotherapy agents, doxorubicin and docetaxel, every 21 days for up to four cycles for the treatment of metastatic or nonmetastatic breast cancer. In this study, 122 patients were randomized to receive a single dose of RYZNEUTA or placebo on Day 2 of chemotherapy cycle 1. All patients received RYZNEUTA on Day 2 of chemotherapy cycles 2 through 4. The benefit of the drug was based upon the mean duration of severe neutropenia in cycle 1, which was lower for RYZNEUTA-treated patients as compared to placebo-treated patients (least-squares mean: 1.4 days versus 4.3 days, respectively, p<0.001 [95% CI: 2.4, 3.5]).
Study GC-627-05 was a randomized, active-controlled, noninferiority study that compared RYZNEUTA to Neulasta. Patients received chemotherapy agents, docetaxel and cyclophosphamide, administered every 21 days for up to four cycles for the treatment of nonmetastatic breast cancer. In this study, 393 patients were randomized to receive a single dose of RYZNEUTA or Neulasta on Day 2 of each chemotherapy cycle. The study demonstrated that the mean days of severe neutropenia of RYZNEUTA-treated patients did not exceed that of Neulasta-treated patients by more than 0.6 days (prespecified noninferiority margin) in cycle 1 of chemotherapy. The mean days of severe neutropenia in cycle 1 was 0.2 days in both the RYZNEUTA and Neulasta arms (difference in means 0.0 days [95% CI: -0.1, 0.1]).
Table 2. Efficacy Results for Clinical Trials GC-627-04 and GC-627-05
| Efficacy Endpoint
|
GC-627-04 (Superiority) |
GC-627-05 (Noninferiority) |
||
|
RYZNEUTA |
Placebo |
RYZNEUTA |
Neulasta |
|
|
Mean duration of severe neutropenia in cycle 1 (SE), days |
1.4 (0.16) |
4.3 (0.24) |
0.2 (0.51) |
0.2 (0.45) |
|
Mean difference (95% CI), days |
2.9 (2.3, 3.4) |
0.0 (-0.1, 0.1) |
||
|
Margin, days |
0 (superiority) |
0.6 (noninferiority) |
||
Source: Adapted from FDA Review
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: RYZNEUTA was only assessed in female patients. It is reasonable to believe that RYZNEUTA works as well in male patients as it does in female patients due to phase 1 studies done in healthy male volunteers.
- Race: Although the clinical trials contained one Black patient, it is reasonable to believe that RYZNEUTA works as well in Black patients as well as it does in White patients because how U.S.-licensed granulocyte colony stimulating factors work have not been shown to be dependent on race in patients.
- Age: RYZNEUTA worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup analyses by sex, race, ethnicity, and geographic region are not applicable for Study GC-627-04 and GC-627-05 due to the homogeneity of the study population.
In Study GC-627-04, all 122 randomized participants were female. Almost all participants were white except for one Black or African American patient in the RYZNEUTA arm. Almost all participants were not Hispanic or Latino except for two participants (one in the RYZNEUTA arm and one in the placebo arm). Almost all were from Eastern Europe (Ukraine, Russia, or Hungary) except for one participant from the United States in the RYZNEUTA arm.
The subgroup analysis by age (<65 years versus ≥65 years) is presented in Table 3 and Table 4. Most participants were in the <65 years age group (91.8%). Therefore, the estimated treatment effect of RYZNEUTA in this subgroup is similar to that of the overall population. The estimated treatment effect of RYZNEUTA in the ≥65 years subgroup may be biased and noninformative because of a wide 95% confident interval due to the small sample size.
Table 3. Subgroup Analysis by Age Group for Trial GC-627-04
|
Mean Duration of Severe Neutropenia in Cycle 1 (Days) |
Mean Difference (Placebo-RYZNEUTA) (95% CI) |
|
Overall result |
2.9 (2.3, 3.4) |
|
Age group, years |
|
|
<65 |
2.6 (2.0, 3.2) |
|
≥65 |
2.1 (0.1, 4.1) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval
Table 4. Subgroup Analysis by Age Group for Trial GC-627-05
|
Mean Duration of Severe Neutropenia in Cycle 1 (Days) |
Mean Difference (RYZNEUTA-Neulasta) (95% CI) |
|
Overall result |
0.0 (-0.1, 0.1) |
|
Noninferiority margin |
0.6 |
|
Age group, years |
|
|
<65 |
0.0 (-0.2, 0.2) |
|
≥65 |
0.5 (-0.8, 1.8) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval
What are the possible side effects?
RYZNEUTA can cause fatal splenic rupture, acute respiratory distress syndrome, serious allergic reactions including anaphylaxis, sickle cell crises in patients with sickle cell disorders, glomerulonephritis, thrombocytopenia, capillary leak syndrome, and myelodysplastic syndrome and acute myeloid leukemia in patients with breast and lung cancer.
The most common side effects of RYZNEUTA are nausea, anemia, and thrombocytopenia.
What are the possible side effects (results of trials used to assess safety)?
Table 5. Adverse Reactions* of Patients Receiving RYZNEUTA, Trial GC-627-04
|
Adverse Reactions |
RYZNEUTA N=83 n (%) |
Placebo N=39 n (%) |
|
Nausea |
42 (51) |
15 (39) |
|
Anemia |
12 (15) |
4 (10) |
|
Thrombocytopenia |
10 (12) |
1 (3) |
Source: RYZNEUTA Prescribing Information
*Adverse reactions that occurred in ≥10% of RYZNEUTA-treated patients and ≥5% more than placebo-treated patients.
Were there any differences in side effects among sex, race, and age?
- Sex: There were no males included in the main trials, therefore, the difference in side effects among males and females cannot be assessed.
- Race: The occurrence of side effects in race could not be determined because the number of patients of races other than White was small.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The safety profile of RYZNEUTA was similar among age groups. There were not enough patients included in the trials to assess a difference in sex and race subgroups.
Table 6. Overview of Adverse Events by Demographic Subgroup
|
Characteristic |
GC-627-04 |
GC-627-05 |
Total RYZNEUTA |
||
|
RYZNEUTA |
Placebo |
RYZNEUTA |
Neulasta |
||
|
Age group, years |
|
|
|
|
|
|
<65 |
68/76 (89.5) |
33/36 (91.7) |
153/171 (89.5) |
136/161 (84.5) |
221/247 (89.5) |
|
≥65 |
6/7 (85.7) |
3/3 (100) |
25/26 (96.2) |
33/35 (94.3) |
31/33 (93.9) |
|
Race |
|
|
|
|
|
|
Black or African American |
1/1 (100) |
0/0 (NA) |
0/0 (NA) |
0/0 (NA) |
1/1 (100) |
|
White |
73/82 (89.0) |
36/39 (92.3) |
178/197 (90.4) |
169/196 (86.2) |
251/279 (90.0) |
|
Ethnicity |
|
|
|
|
|
|
Hispanic or Latino |
1/1 (100) |
1/1 (100) |
0/0 (NA) |
1/1 (100) |
1/1 (100) |
|
Not Hispanic or Latino |
73/82 (89.0) |
35/38 (92.1) |
178/197 (90.4) |
168/195 (86.2) |
251/279 (90.0) |
Source: Adapted from FDA Review
Abbreviations: N, number of patients in treatment arm; n, number of patients with adverse event; NA, not applicable; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION