Drug Trials Snapshots: POMBILITI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The "MORE INFO" bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the POMBILITI Prescribing Information and Opfolda (drug used in combination with POMBILITI) Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
POMBILITI (cipaglucosidase alfa-atga)
pom-BILL-ih-tee
Amicus Therapeutics US, Inc.
Original Approval date: September 28, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
POMBILITI is an enzyme used for the treatment of adult patients with late-onset Pompe disease (LOPD) weighing ≥40 kg and who are not improving on their current enzyme replacement therapy. POMBILITI is approved for use in combination with Opfolda.
How is this drug used?
POMBILITI is given by a healthcare professional through a needle placed in a vein (known as intravenous infusion) every two weeks. Approximately one hour before receiving each infusion of POMBILITI, patients take one dose of Opfolda by mouth.
Who participated in the clinical trials?
The FDA approved POMBILITI in combination with Opfolda based on evidence from a clinical trial (Trial 1/NCT03729362) of 123 patients with LOPD. The trial was conducted at 61 sites in 24 countries around the world, including the United States.
Safety data from the use of POMBILITI in combination with Opfolda was primarily obtained from one clinical trial (Trial 1, NCT03729362). Data from two other trials (Trial 2/NCT02675465 and Trial 3/NCT04138277) were also reviewed for completeness of the safety assessment. The three trials enrolled 151 patients with LOPD. The trials were conducted at 61 sites in 24 countries around the world, including the United States.
How were the trials designed?
POMBILITI in combination with Opfolda was evaluated in three clinical trials of 151 adult patients with LOPD. Trial 1 evaluated the benefits and side effects of POMBILITI in combination with Opfolda, and all three trials evaluated the side effects of POMBILITI in combination with Opfolda.
In Trial 1, 123 adult patients with LOPD received either POMBILITI intravenously once every 2 weeks for 52 weeks in combination with Opfolda, or another drug (called the active comparator) intravenously once every 2 weeks for 52 weeks in combination with placebo. Of the 123 patients, 95 previously received enzyme replacement therapy, and 28 never received enzyme replacement therapy before the trial. Neither the patients nor the healthcare providers knew which treatment was being given until after Week 52.
The benefit of POMBILITI in combination with Opfolda was evaluated by comparing the change in lung function between patients who received POMBILITI in combination with Opfolda to the change in patients who were treated with the active comparator.
How were the trials designed?
The efficacy and safety of POMBILITI in combination with Opfolda were evaluated in Trial 1 of 123 adult patients with LOPD. The safety of POMBILITI in combination with Opfolda was also evaluated in two additional trials of patients with LOPD.
Trial 1 was a randomized, double-blind, active comparator-controlled trial where 85 patients received 20 mg/kg of POMBILITI intravenously in combination with Opfolda once every 2 weeks for 52 weeks and 38 patients received active comparator intravenously in combination with placebo once every 2 weeks for 52 weeks. Trial 1 was followed by an open-label trial in which all patients from Trial 1 received POMBILITI in combination with Opfolda for up to two years. The open-label trial is ongoing.
The benefit of POMBILITI in combination with Opfolda was evaluated by comparing the change in forced vital capacity (% predicted) between patients who received POMBILITI in combination with Opfolda to the change in patients who were treated with the active comparator.
DEMOGRAPHICS SNAPSHOT:
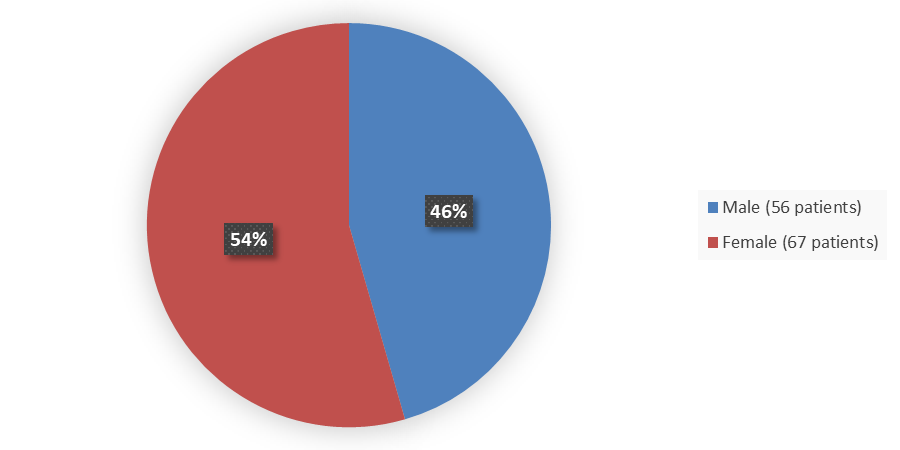
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy of POMBILITI in combination with Opfolda.
Figure 1. Baseline Demographics by Sex (Efficacy Population – Trial 1)
Source: Adapted from FDA Review
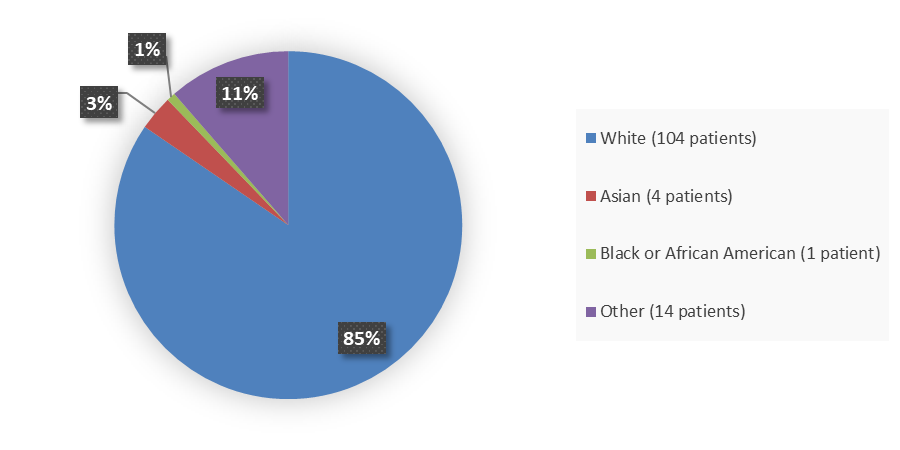
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy of POMBILITI in combination with Opfolda.
Figure 2. Demographics by Race (Efficacy Population – Trial 1)
Source: Adapted from FDA Review
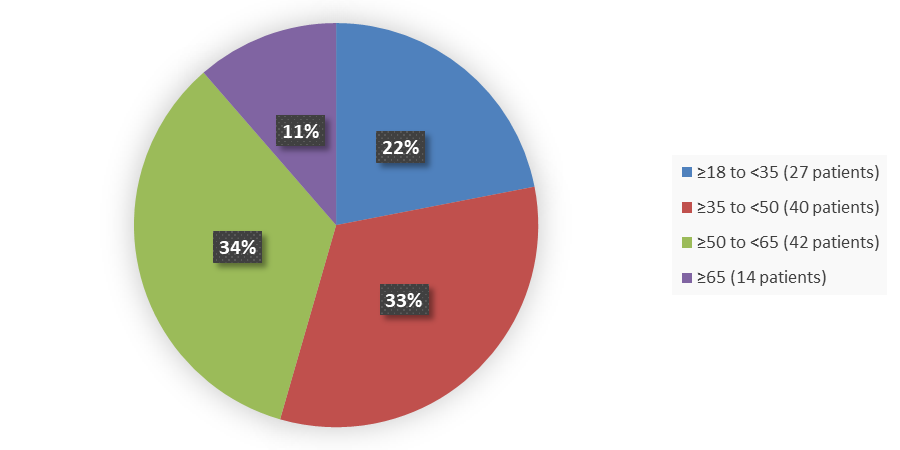
Figure 3 summarizes the percentage of patients by age in the clinical trial used to evaluate the efficacy of POMBILITI in combination with Opfolda.
Figure 3. Baseline Demographics by Age (Efficacy Population – Trial 1)
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 summarizes the demographics of patients in the efficacy trial population and the pooled safety trial population.
Table 1. Baseline Demographics of Enrolled Patients in the Clinical Trials
| Demographic |
Efficacy Population Trial 1 N=123 |
Safety Population Trials 1, 2, 3 N=151 |
|
Sex, n (%) |
|
|
|
Female |
67 (54.5) |
80 (53.0) |
|
Male |
56 (45.5) |
71 (47.0) |
|
Race, n (%) |
|
|
|
Asian |
4 (3.3) |
4 (2.6) |
|
Black or African American |
1 (0.8) |
1 (0.7) |
|
Multiple |
14 (11.4) |
13 (8.6) |
|
Not reported |
0 (0) |
12 (7.9) |
|
White |
104 (84.6) |
121 (80.1) |
|
Age. years |
|
|
|
Mean (SD) |
46.8 (13.3) |
46.9 (13.4) |
|
Median |
47 |
48 |
|
Min, max |
19, 74 |
18, 74 |
|
Age group, years, n (%) |
|
|
|
≥18 to <35 |
27 (22.0) |
32 (21.2) |
|
≥35 to <50 |
40 (32.5) |
49 (32.5) |
|
≥50 to <65 |
42 (34.1) |
53 (35.1) |
|
≥65 |
14 (11.4) |
17 (11.3) |
|
Region, n (%) |
|
|
|
United States |
37 (30.1) |
53 (35.1) |
|
Rest of the world |
86 (69.9) |
98 (64.9) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
What are the benefits of this drug?
Like the active comparator drug, POMBILITI in combination with Opfolda improved lung function in adult patients with LOPD after 52 weeks of treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 summarizes the efficacy results in sitting forced vital capacity (% predicted) at Week 52 for Trial 1.
Table 2. Summary of Sitting FVC in Adults With LOPD by ERT Status at 52 Weeks in Trial 1
|
Sitting FVC (% Predicted) |
ERT-Experienced |
ERT-Naïvea |
||
|
POMBILITI + Opfolda |
Active Comparatorb + Placebo |
POMBILITI + Opfolda |
Active Comparatorb + Placebo |
|
|
Baseline |
|
|
|
|
|
n |
65 |
30 |
20 |
8 |
|
Mean (SD) |
67.9 (19.1) |
67.5 (21.0) |
80.2 (18.7) |
79.6 (21.0) |
|
Median |
68.0 |
69.0 |
82.3 |
88.5 |
|
Change from baseline at Week 52 |
|
|
|
|
|
n |
55 |
26 |
19 |
7 |
|
Mean (SD) |
0.1 (5.9) |
-3.5 (4.7) |
-4.7 (6.2) |
-2.4 (6.3) |
|
Median |
0.5 |
-2.5 |
-4.5 |
-3.0 |
|
Change to Week 52 |
|
|
||
|
Difference of means (SE) |
3.5 (1.3) |
-1.9 (2.7) |
||
|
(95% CI) |
(1.0, 6.0)c |
(-7.3, 3.6) |
||
Source: POMBILITI Prescribing Information
a POMBILITI in combination with Opfolda is not approved for use in ERT-naïve patients with LOPD. The ERT naïve patient subgroup enrolled too few patients to conclusively interpret the data. For the ERT-naïve group, the treatment difference was estimated using a 2-sample t-test.
bA U.S.-approved alglucosidase alfa product was not used in this clinical trial. Conclusions cannot be drawn from this clinical trial regarding comparative effectiveness between a U.S.-approved alglucosidase alfa product and POMBILITI in combination with Opfolda for the treatment of adult patients with LOPD weighing ≥40 kg and who are not improving on their current ERT.
c For the ERT-experienced group, the treatment difference of the mean was estimated by analysis of covariance which included treatment, gender, baseline FVC, age, weight, and height in the model. Nominal p=0.006. Missing data at Week 52 was imputed using last observed values.
Abbreviations: CI, confidence interval; ERT, enzyme replacement therapy; FVC, forced vital capacity; LOPD, late-onset Pompe disease; SD, standard deviation; SE, standard error
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: POMBILITI in combination with Opfolda worked similarly in males and females.
- Race: The majority of patients in the trials were White. Differences in response to POMBILITI in combination with Opfolda among races could not be determined.
- Age: The majority of the patients were younger than 65 years of age. Differences in how well POMBILITI in combination with Opfolda worked between those younger and older than 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 summarizes subgroup analyses by race, age, and sex. However, the trial population was too small to support reliable interpretation of these subgroup analyses.
Table 3. Subgroup Analysis of Change From Baseline to Week 52 of Sitting FVC in Adults With LOPD in Trial 1
|
Subgroup |
POMBILITI + Opfolda N/Mean |
Active Comparatora + Placebo N/Mean |
Difference (95% CI) |
|
Overall |
84/-1.1 |
38/-3.4 |
2.3 (0.0, 4.6) |
|
Sex |
|
|
|
|
Female |
49/-0.5 |
18/-4.4 |
3.9 (0.5, 7.3) |
|
Male |
35/-2.0 |
20/-2.1 |
0.2 (-3.2, 3.5) |
|
Age, years |
|
|
|
|
<45 |
35/-1.0 |
17/-3.3 |
2.3 (-1.3, 5.9) |
|
≥45 |
49/-1.0 |
21/-3.6 |
2.6 (-0.6, 5.7) |
|
Race |
|
|
|
|
White |
73/-1.5 |
30/-3.5 |
2.1 (-0.5, 4.6) |
|
Other |
11/0.9 |
8/-1.5 |
2.4 (-3.4, 8.2) |
Source: Adapted from FDA Review
a A U.S.-approved alglucosidase alfa product was not used in this clinical trial. Conclusions cannot be drawn from this clinical trial regarding comparative effectiveness between a U.S.-approved alglucosidase alfa product and POMBILITI in combination with Opfolda for the treatment of adult patients with LOPD weighing ≥40 kg and who are not improving on their current ERT.
Abbreviations: CI, confidence interval; ERT, enzyme replacement therapy; FVC, forced vital capacity; LOPD, late-onset Pompe disease
What are the possible side effects?
POMBILITI in combination with Opfolda may cause serious side effects including life-threatening allergic reactions during and after the infusion and harm to an unborn baby if taken while pregnant.
The most common side effects of POMBILITI in combination with Opfolda are headache, diarrhea, fatigue, nausea, abdominal pain, and fever.
What are the possible side effects (results of trials used to assess safety)?
Table 4 summarizes adverse reactions observed in patients during Trial 1.
Table 4. Adverse Reactions That Occurred in Adults With LOPD at an Incidence of ≥2% in Trial
|
Adverse Reaction |
POMBILITI + Opfolda N=85 n (%) |
Active Comparatora + Placebo N=38 n (%) |
|
Headacheb |
7 (8.2) |
3 (7.9) |
|
Diarrhea |
5 (5.9) |
2 (5.3) |
|
Dizziness |
4 (4.7) |
2 (5.3) |
|
Dyspnea |
3 (3.5) |
0 |
|
Abdominal distention |
3 (3.5) |
2 (5.3) |
|
Pyrexia |
3 (3.5) |
1 (2.6) |
|
Rashc |
3 (3.5) |
0 |
|
Abdominal paind |
2 (2.4) |
4 (10.5) |
|
Nausea |
2 (2.4) |
5 (13.2) |
|
Chills |
2 (2.4) |
0 |
|
Dysgeusia |
2 (2.4) |
0 |
|
Flushing |
2 (2.4) |
0 |
|
Muscle spasms |
2 (2.4) |
0 |
|
Pruritus |
2 (2.4) |
2 (5.3) |
|
Tachycardiae |
2 (2.4) |
0 |
|
Urticariaf |
2 (2.4) |
0 |
Source: POMBILITI Prescribing Information
a A U.S.-approved alglucosidase alfa product was not used in this clinical trial. Conclusions cannot be drawn from this clinical trial regarding comparative effectiveness between a U.S.-approved alglucosidase alfa product and POMBILITI in combination with Opfolda for the treatment of adult patients with LOPD weighing ≥40 kg and who are not improving on their current ERT.
b Headache included migraine and migraine with aura.
c Rash included erythematous rash and macular rash.
d Abdominal pain included upper and lower abdominal pain.
e Tachycardia included sinus tachycardia.
f Urticaria included mechanical urticaria and urticarial rash.
Abbreviations: ERT, enzyme replacement therapy; LOPD, late-onset Pompe disease
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The majority of patients in the trials were White. Differences in side effects among races could not be determined.
- Age: The majority of the patients were younger than 65 years of age. Differences in the occurrence of side effects between those younger and older than 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The trial population was too small to support reliable interpretation of the subgroup analyses by race, age, and sex.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
LINK TO DRUG PACKAGE INSERT: