Temporary Tattoos, Henna/Mehndi, and "Black Henna": Fact Sheet
Safety and Regulatory Information
Quick Guide
Learn the fast facts about types of tattoos, risks to consider, removals, and FDA’s role in monitoring safety.
- Tattoos & Permanent Makeup: Quick Guide (PDF: 536 KB)
- Los Tatuajes y el Maquillaje Permanente: Una Guía (PDF: 522KB)
FDA has received reports of adverse reactions to some “decal,” henna, and “black henna” temporary tattoos. Here is information about the safety of these products and how they are regulated.
- "Decal" Temporary Tattoos
- Henna, or Mehndi, and "Black Henna"
- Finding out What's in a Temporary Tattoo or Henna/Mehndi Product
- FDA's Authority over Color Additives in Cosmetics
- FDA's Authority over Other Cosmetic Ingredients
- FDA Enforcement Action
- How to Report a Reaction to a Temporary Tattoo or Other Cosmetic
- More Resources on Temporary Tattoos:
- Related Resources:
"Decal" Temporary Tattoos
Decal temporary tattoos are used to decorate any part of the body, including areas of the face and around the eyes, and may last for a day or up to a week or more. They are especially popular with children and at Halloween.
There are two kinds of decal tattoos:
- Some are images attached to a removable backing. The decal image is removed from the backing by wetting, and the image is then applied directly to the skin.
- Others have a backing that adheres to the skin, creating a partial or complete barrier between the skin and the dyes used in the image.
The difference is important, because not all dyes are known to be safe for use on the skin. While an adhesive backing may protect the skin from unapproved colors, there may be other ingredients on or in the decal to help the image adhere better either to the backing or to the skin that may cause problems for some people.
FDA has received reports of reactions to some decal-type temporary tattoos. Before using a temporary tattoo on your face, it may be a good idea to try it on a less conspicuous part of your body.
Henna, or Mehndi, and "Black Henna"
Henna, a coloring made from a plant, is approved only for use as a hair dye. It is not approved for direct application to the skin, as in the body-decorating process known as mehndi. This unapproved use of a color additive makes these products adulterated. It is unlawful, for example, to introduce an adulterated cosmetic into interstate commerce.
Because henna typically produces a brown, orange-brown, or reddish-brown tint, other ingredients must be added to produce other colors, such as those marketed as "black henna" and "blue henna." Even brown shades of products marketed as henna may contain other ingredients intended to make them darker or make the stain last longer on the skin.
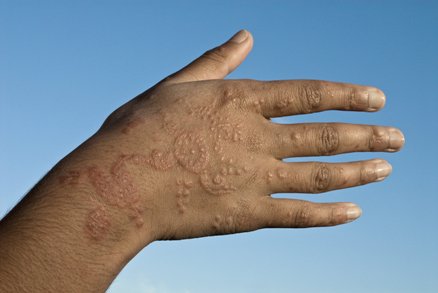
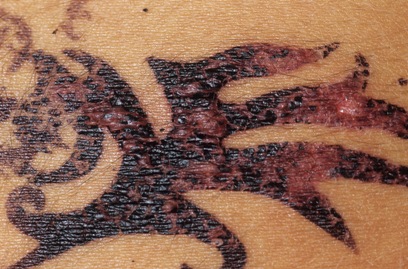
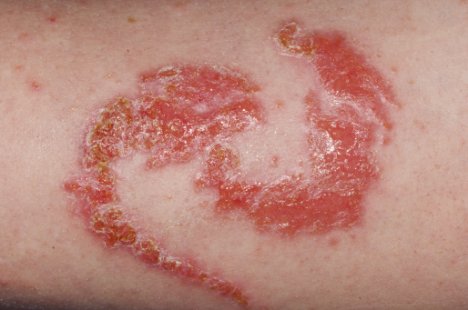
The extra ingredient used to blacken henna is often a coal-tar hair dye containing p-phenylenediamine (PPD), an ingredient that can cause dangerous skin reactions in some people. That's the reason hair dyes have a caution statement and instructions to do a "patch test" on a small area of the skin before using them. Sometimes, the artist may use a PPD-containing hair dye alone. Either way, there's no telling who will be affected. By law, PPD is not permitted in cosmetics intended to be applied to the skin.
FDA has received reports of injuries to the skin from products marketed as henna and products marketed as "black henna." For more information on Henna, see the consumer update: Temporary Tattoos May Put You at Risk.
Allergic reaction on a man's hand. J. Cole/Photo Researchers.
Allergic reaction on a 14-year-old girl. Dr. P. Marazzi/Photo Researchers.
Allergic reaction on an arm. Dr. P. Marazzi/Photo Researchers.
Finding out What's in a Temporary Tattoo or Henna/Mehndi Product
Cosmetics that are sold on a retail basis to consumers must have their ingredients listed on the label. Without such an ingredient declaration, they are considered misbranded, and therefore it is unlawful to introduce them into interstate commerce. FDA requires the ingredient declaration under the authority of the Fair Packaging and Labeling Act (FPLA).
Because the FPLA does not apply to cosmetic samples and products used only by professionals--for example, for application at a salon, or a booth at a fair or boardwalk--the requirement for an ingredient declaration does not apply to these products.
FDA's Authority over Color Additives in Cosmetics
By law, all color additives used in cosmetics must be approved by FDA for their intended uses, with the exception of coal tar colors intended for use in hair dyes. In addition, some color additives must not be used unless FDA has certified that the batch meets the regulatory requirements for composition and purity. Cosmetics, including temporary tattoo products, that do not comply with restrictions on color additives are considered adulterated, and it is unlawful to introduce them into interstate commerce. To learn more, see Color Additives and Cosmetics, and, for information on how color additives are approved, Color Additive Petitions.
FDA's Authority over Other Cosmetic Ingredients
Cosmetics must be safe when consumers use them following directions on the label, or in the customary or expected way. Except for color additives, the law does not require cosmetic products and ingredients to have FDA approval before they are marketed. In addition, firms are not required to report their safety information, including complaints.
For a list of ingredients that are prohibited or restricted in cosmetics, see “Prohibited and Restricted Ingredients.”
For a list of color additives allowed in cosmetics, how they are allowed to be used, and links to their regulations, see “Color Additives Permitted for Use in Cosmetics.”
FDA Enforcement Action
FDA can take action against cosmetics on the market that don’t comply with the law. For example, we can issue Import Alerts and Warning Letters.
An Import Alert allows FDA to detain products that violate or appear to violate the Federal Food, Drug, and Cosmetic Act. We have two Import Alerts in effect for temporary tattoos. However, because not all shipments of imported cosmetics are inspected, it’s still possible for some unsafe or mislabeled products to be imported into this country.
- An Import Alert is in effect for several foreign-made decal temporary tattoos because they contain colors not permitted for use in cosmetics applied to the skin, or they don't have the required ingredient list on the label, or they are labeled as “FDA approved.”
- An Import Alert is in effect for henna intended for use on the skin because it is an unapproved use of the color additive.
FDA issues Warning Letters to let companies know that they have violated the law and to tell them what corrective action they need to take. We have issued a Warning Letter to a company marketing “black henna” products:
It is important to note that the practice of tattooing is generally regulated by state and/or local officials, and that the FDA is not typically involved in such enforcement. While states have jurisdiction over professional practices such as tattooing and cosmetology, that oversight differs from state to state. Some states have laws and regulations for temporary tattooing, while others don't. So, depending on where you are, it's possible no one is checking to make sure the artist is following safe practices or even knows what may be harmful to consumers.
How to Report a Reaction to a Temporary Tattoo or Other Cosmetic Products
It’s important for consumers and health care professionals to report problems with cosmetic products to FDA. This information helps FDA find out which products are causing problems, and what kinds of problems.
You can report a problem with a cosmetic product to FDA by using the contact information at How to Report a Cosmetic Product Related Complaint | FDA.
Related Resources
- Consumer Update: Temporary Tattoos May Put You at Risk
- Temporary Tattoos: Raising Consumer Awareness of Safety: FDA Webinar, May 13, 2014
- Warning Letter Issued to Black Henna Ink, Inc.
- Import Alert #53-19: Detention Without Physical Examination of Henna Based Skin Color
- Lucky 13 Tips for a Safe Halloween
- Novelty Makeup: Special for Halloween
- Tattoos and Permanent Makeup
- FDA Authority Over Cosmetics
- FDA Recall Policy for Cosmetics
- Tattoos & Permanent Makeup: Quick Guide (PDF: 536 KB)
- Los Tatuajes y el Maquillaje Permanente: Una Guía (PDF: 522KB)