Cigarette Labeling and Health Warning Requirements
On this page:
- Cigarette Health Warnings
- Cigarette Packages
- Cigarette Advertisements
- Cigarette Plans for Required Warnings
Cigarette Health Warnings
The Family Smoking Prevention and Tobacco Control Act (TCA) granted FDA important new authority to regulate the manufacture, marketing, and distribution of tobacco products. The TCA also amended Section 4 of the Federal Cigarette Labeling and Advertising Act (FCLAA), directing FDA to issue regulations requiring color graphics depicting the negative health consequences of smoking to accompany new textual warning statements. The TCA amends the FCLAA to require each cigarette package and advertisement to bear one of the new required warnings.
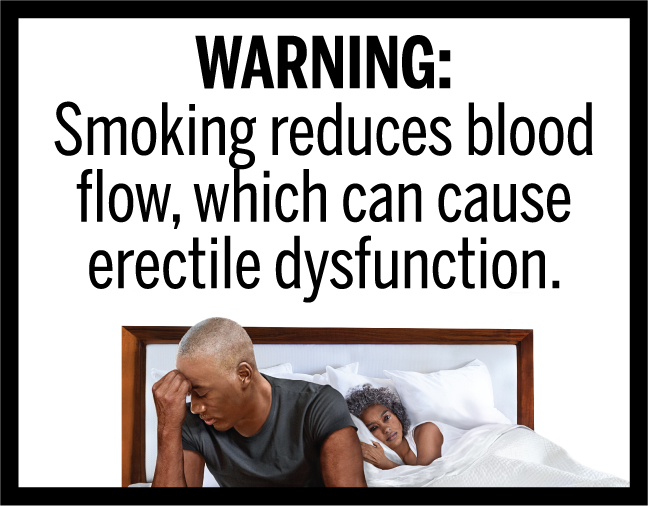
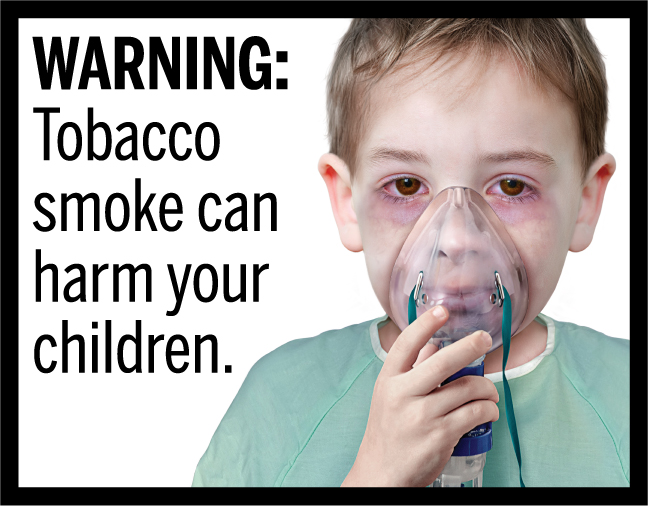
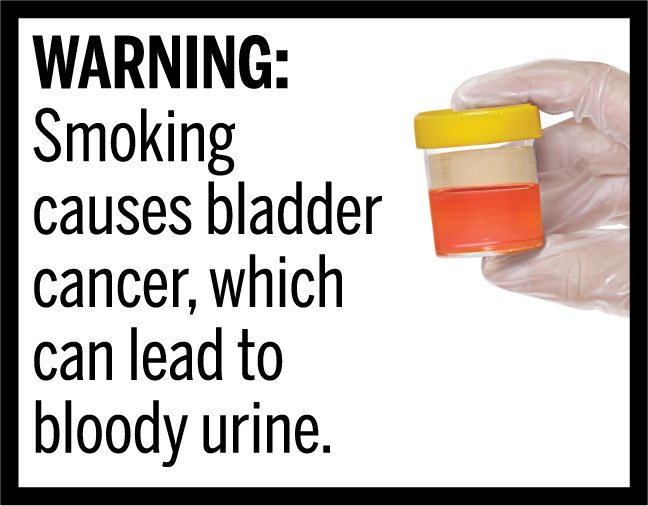
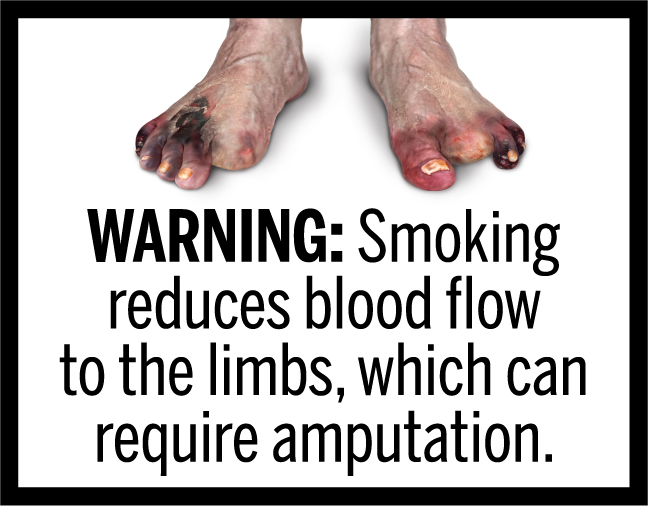
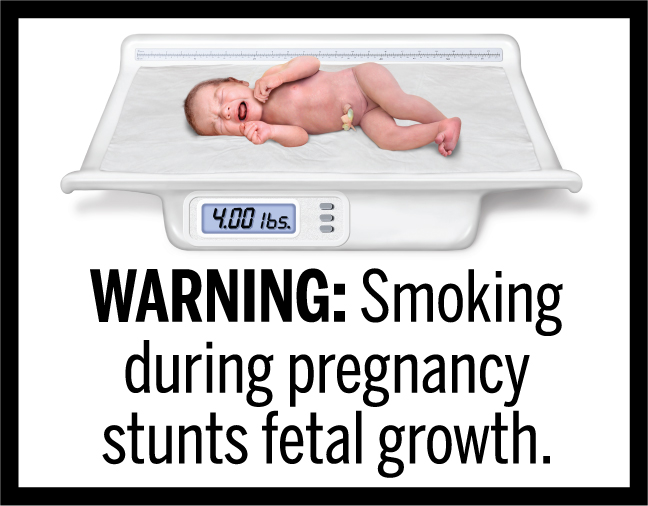
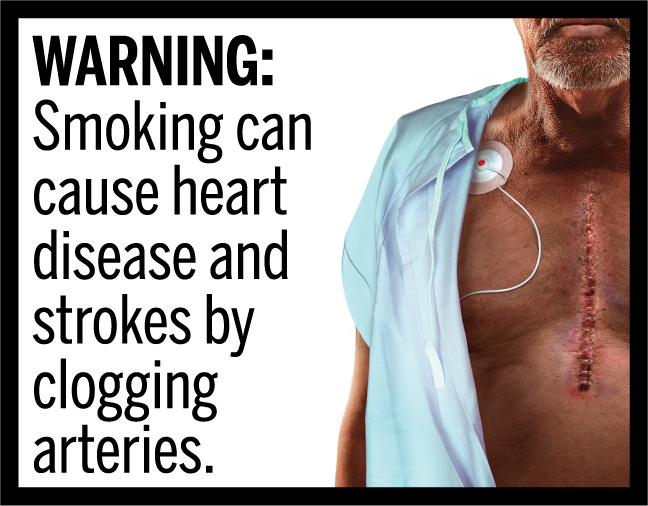
In March 2020, FDA finalized the “Required Warnings for Cigarette Packages and Advertisements” rule, establishing 11 new cigarette health warnings, consisting of textual warning statements accompanied by color graphics, in the form of concordant photorealistic images, depicting the negative health consequences of cigarette smoking. These new required warnings depict some of the lesser-known, but serious health risks of smoking.
FDA has also published the “Required Warnings for Cigarette Packages and Advertisements – Small Entity Compliance Guide” to help small businesses understand and comply with the final rule.
Current Status of Final Rule
On Dec. 7, 2022, the U.S. District Court for the Eastern District of Texas issued an order in R.J. Reynolds Tobacco Co. et al. v. United States Food and Drug Administration et al., No. 6:20-cv-00176, vacating the “Required Warnings for Cigarette Packages and Advertisements” final rule. On March 21, 2024, the U.S. Court of Appeals for the Fifth Circuit issued an opinion reversing the District Court and concluding that FDA’s rule is consistent with the First Amendment. The opinion remanded the case to the District Court for consideration of plaintiffs’ remaining claims. Plaintiffs filed a motion for interim relief on the remanded claims. On January 14, 2025, the District Court entered a preliminary injunction and postponed the rule’s effective date until entry of final judgment in the litigation. FDA is appealing the District Court’s decision.
On August 29, 2025, the U.S. District Court for the Southern District of Georgia issued an order in Philip Morris USA Inc. et al. v. FDA, 2:24-cv-00143, vacating and setting aside the final rule.
Guidance to Industry
On September 12, 2024, FDA issued guidance for industry that describes the agency’s enforcement policy for the final rule. However, more recently, as described above, two District Courts have issued orders preventing FDA from enforcing the rule. Litigation remains pending.
Required Warnings for Cigarette Packages and Advertisements
See FDA’s Cigarette Health Warning Design Files and Technical Specifications to download the design files and for further instructions.
Interactive Cigarette Health Warning
Use your mouse to rotate this 3D image of one of FDA’s new proposed cigarette health warnings to see what it would look like on a cigarette package. You can also download this interactive image, and share it on your digital space.
Cigarette Packages
- Size and location – The required warning must comprise at least the top 50 percent of the front and rear panels of the cigarette package (i.e., the two largest sides or surfaces of the package).
For cigarette cartons, the required warnings must be located on the left side of the front and rear panels of the carton and must comprise at least the left 50 percent of these panels. The required warning must appear directly on the package and must be clearly visible underneath any cellophane or other clear wrapping. - Orientation – The required warning must be positioned so that the text of the required warning and the other information on that panel of the package have the same orientation.
For example, if the front panel of a cigarette package contains information, such as the brand name of the cigarette, in a left to right orientation, the required warning, including the textual warning statement, must also appear in a left to right orientation. - Random and equal display and distribution – All 11 required warnings for packages must be randomly displayed in each 12-month period, in as equal a number of times as is possible on each brand of the product and must be randomly distributed in all areas of the United States in which the product is marketed, in accordance with an FDA-approved cigarette plan.
- Irremovable or permanent warnings – Required warnings must be indelibly printed on or permanently affixed to the cigarette package.
For example, these required warnings must not be printed or placed on a label affixed to a clear outer wrapper that is likely to be removed to access the product within the package.
Cigarette Advertisements
- Size and location – For print advertisements and other advertisements with a visual component (including, for example, advertisements on signs, retail displays, Internet web pages, social media web pages, digital platforms, mobile applications, and email correspondence), the required warning must appear directly on the advertisement. Additionally, required warnings must comprise at least 20 percent of the area of the advertisement in a conspicuous and prominent format and location at the top of each advertisement within the trim area, if any.
- Rotation – The 11 required warnings must be rotated quarterly, in alternating sequence, in advertisements for each brand of cigarettes, in accordance with an FDA-approved cigarette plan.
- Irremovable or permanent warnings – Required warnings must be indelibly printed on or permanently affixed to a cigarette advertisement.
Cigarette Plans for Required Warnings
Section 4 of the FCLAA, as amended by the TCA, and the final rule require manufacturers, distributors, and retailers of cigarettes to submit a plan for the random and equal display and distribution of required warnings on cigarettes packages and the quarterly rotation of required warnings in cigarette advertisements, and to obtain FDA approval of their plans before products required to bear such warnings enter the market.
FDA has issued the “Submission of Plans for Cigarette Packages and Cigarette Advertisements (Revised)" guidance to assist those submitting cigarette plans for cigarette packages and advertisements.
The requirement for submission of cigarette plans for cigarette packages and advertisements, and the specific requirements relating to the random and equal display and distribution of the required warnings on cigarette packaging and the quarterly rotation of required warnings in cigarette advertising, appear at Section 4(c) of the FCLAA and 21 CFR 1141.10.
In addition, under Section 201(c) of the TCA and 21 CFR 1141.10(g), the agency must review and approve cigarette plans in advance of any person displaying or distributing packages or advertisements for products that are required to carry the required warnings.
Early submission of cigarette plans will facilitate timely FDA review prior to the effective date of the required warnings, encourage dialogue with entities regarding any implementation concerns, and provide time to consider proposals by entities in a timely manner.
FDA will ensure that its review of cigarette plans will be completed no later than 6 months after receipt of an adequate plan from persons who work in good faith with FDA to complete its review (e.g., persons should work diligently with FDA and be responsive by submitting any requested information in a timely manner).
If a higher volume of submissions is received than is currently expected, FDA intends to ensure that entities who submit an adequate plan by the recommended submission date and who work in good faith with FDA to complete its review are not delayed or prevented from distributing or advertising cigarette packages or advertisements due to the agency not having approved their plans by the effective date of the final rule.
For efficiency of review, FDA recommends that, to the extent possible, manufacturers, distributors, and retailers submit a single cigarette plan that covers both cigarette packaging and cigarette advertising, rather than submitting each plan separately, when applicable.
For FDA to approve a cigarette plan for cigarette packaging, the plan must provide that all of the required warnings are randomly displayed during each 12-month period on each brand of the product, displayed on each brand of the product in as equal a number of times as is possible during each 12-month period, are randomly distributed in all areas of the United States in which the product is marketed, and must ensure that all of the required warnings will be displayed by the manufacturer, distributor, or retailer at the same time.
For FDA to approve a cigarette plan for cigarette advertising, the plan must provide that all of the required warnings are rotated quarterly in alternating sequence in advertisements for each brand of cigarettes.
Additional Resources
- Required Warnings for Cigarette Packages and Advertisements – Small Entity Compliance Guide
- Submission of Plans for Cigarette Packages and Cigarette Advertisements
- Health Warning Design Files and Technical Specifications
- Required Warnings for Cigarette Packages and Advertisements - Final Rule
- Press Release: FDA Requires New Health Warnings for Cigarette Packages and Advertisements