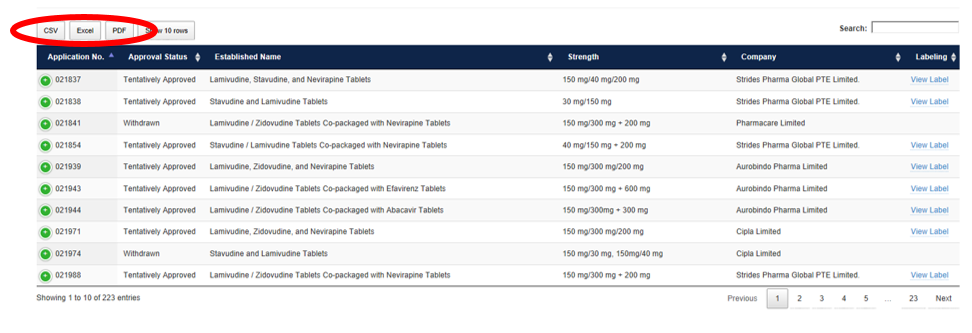
Quick Reference Guide for PEPFAR Database
Interactive database for antiretroviral (ARV) drugs tentatively approved or approved that are eligible for procurement
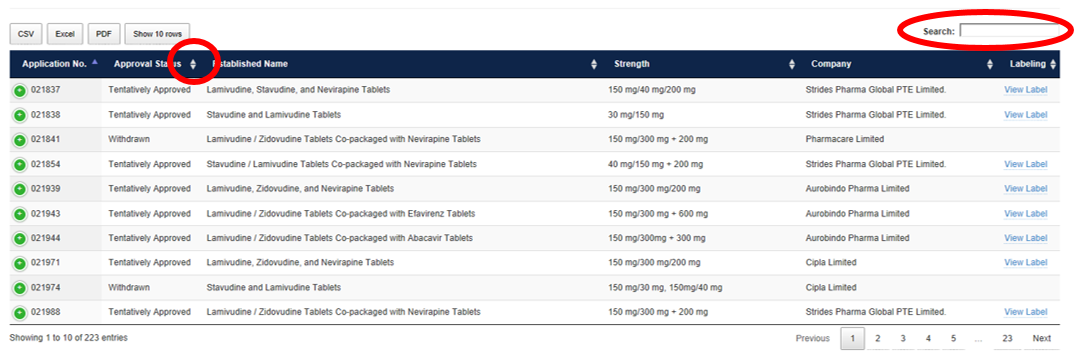
How to Reorder and Filter
Click any carrot to reorder the data. For example, click the carrot next to Approval Status to bring all Approved products to the top or click again to bring all Withdrawn products to the top.
The display can also be filtered by entering text into the Search field. For example, type “Pediatric” and press enter to show only products approved for use in pediatric patients.
The data can be exported in CSV, Excel, or PDF format and filters may additionally be applied in those programs.
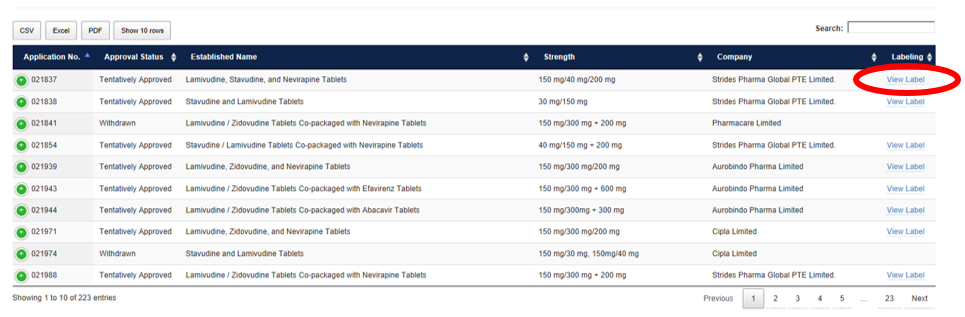
How to Access the FDA-reviewed Labeling
Click “View Label” under Labeling in the far-right column. The FDA-reviewed labeling will open in a new tab or window.
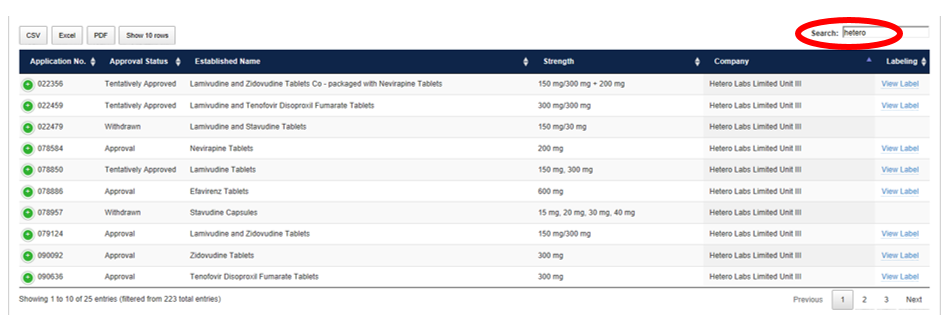
How to Filter by Multiple Terms
Enter multiple keywords to narrow the search results further. For example, search “Hetero” to find all PEPFAR products sponsored by Hetero Labs Limited Unit III.
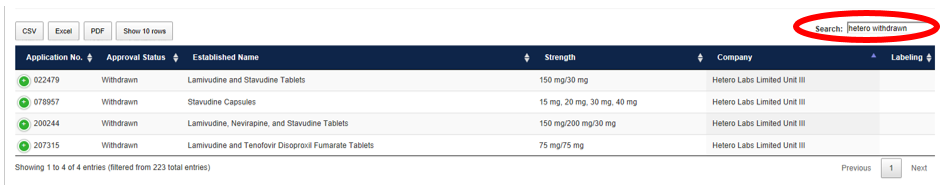
Add “Withdrawn” to the existing search to narrow the results further to display only withdrawn products sponsored by Hetero Labs Limited Unit III.
How to Download Data Files
Search results can be downloaded and saved in CSV, Excel, or PDF format. The database may be downloaded in full or exported containing only the data desired based on the search terms provided.