Want to Quit Smoking? FDA-Approved and FDA-Cleared Cessation Products Can Help
How to Choose a Smoking Cessation Product That’s Right for You
Smoking cessation products approved or cleared by the U.S. Food and Drug Administration are shown to help people quit smoking and can even double your chance of quitting successfully.
Are you or a loved one among those American adults who smoke cigarettes and want to quit? Whether this is your first attempt to quit smoking, or you’ve already tried several times, know that there are FDA-approved and FDA-cleared products that can assist you on your journey to becoming smoke-free.
If you’re considering using a smoking cessation product and are under age 18, speak to a doctor before using these products.
Benefits of Quitting Smoking
No matter how much you smoke—or for how long—quitting will benefit you.
Quitting smoking can lower risk of:
- Various cancers, including lung cancer
- Heart disease, stroke, emphysema, vision loss, and other serious diseases
- Disease and cancer in family members including children, and pets, who otherwise would be exposed to your secondhand smoke
Although there are benefits to quitting at any age, it is important to quit as soon as possible so your body can begin to recover from the damage caused by smoking. For instance, on average, 12 hours after you quit smoking the carbon monoxide level in your blood drops to normal. Carbon monoxide is harmful because it displaces oxygen in the blood and deprives your heart, brain, and other vital organs of oxygen.
Don’t know what to expect when you first quit smoking? Learn more about what it’s like to quit smoking.
What is Nicotine Replacement Therapy (NRT)?
Nicotine is a highly addictive chemical compound present in a tobacco plant. Tobacco products are addictive because they contain nicotine. Nicotine keeps people using tobacco products, even when they want to stop.
Nicotine replacement therapy, also known as NRT, helps you quit smoking by gradually providing the body with smaller doses of nicotine over time, without exposing you to the toxic chemicals found in cigarette smoke. In conjunction with a behavioral program, NRTs have been found to increase the success of smoking cessation and are available over-the-counter and by prescription.
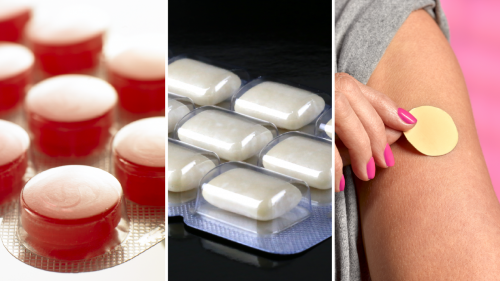
Over-the-counter NRTs are approved for sale to people age 18 and older. They include:
- Skin patches (also called “transdermal nicotine patches”). These patches are placed on the skin, similar to how you would apply an adhesive bandage.
- Chewing gum (also called “nicotine gum”). This gum must be chewed according to the labeled instructions to be effective.
- Lozenges (also called “nicotine lozenges”). You use these products by dissolving them in your mouth.
Prescription Smoking Cessation Products That Contain Nicotine
- Nicotine spray
- Nicotine inhaler
Prescription Smoking Cessation Products That Do Not Contain Nicotine
Two FDA-approved smoking cessation products do not contain nicotine:
- Varenicline tartrate
- Bupropion hydrochloride
Both are available in tablet form by prescription only. The FDA evaluated these drugs and found that the benefits outweigh the risks. For users taking these products, risks include changes in behavior, depressed mood, hostility, aggression, and suicidal thoughts or actions.
The most common side effects of varenicline tartrate include nausea; constipation; gas; vomiting; and trouble sleeping or vivid, unusual, or strange dreams. Varenicline tartrate also may change how you react to alcohol, so talk to your health care provider about your drinking habits (if you drink alcohol) and whether these habits need to change. Varenicline tartrate is not recommended for use in patients 16 years of age or younger.
The most commonly observed side effects consistently associated with the use of bupropion hydrochloride are dry mouth and insomnia. Because bupropion hydrochloride contains the same active ingredient as the antidepressant bupropion, the FDA encourages people who use bupropion hydrochloride —and those who are considering it—to talk to their health care providers about the risks of treatment with antidepressant drugs. Bupropion hydrochloride has not been studied in children under the age of 18 and is not approved for use in children and teenagers.
Other Smoking Cessation Therapies
The FDA has also given marketing clearance to a device using Transcranial Magnetic Stimulation (TMS) as an aid for short-term smoking cessation in adults.
Talk to Your Health Care Provider
Talk to your health care provider to determine the best course of treatment and which smoking cessation products might work best for you. Your health care provider may need to take into consideration your age, whether you’re pregnant or breastfeeding, and medical history, such as heart disease, diabetes, depression, asthma, blood pressure, and more.
You can also read the labels to better understand how the products work and what side effects they may cause. If you want to know more about a specific drug, you can search the FDA's Drugs@FDA website, which includes information on each product by name. If you want to know more about a specific device, you can search the FDA’s Product Classification Database, for “smoking cessation” medical devices.
Discuss the possible side effects with your health care provider and call them if you experience side effects as they may tell you to stop using the product. If you have any side effects related to any smoking cessation products, or any other problems related to your treatment, the FDA would like to hear from you. Please consider making a voluntary and confidential report to FDA’s MedWatch program. You can also watch this short video to find out more about submitting a report.
