FDA's Approach to the GRAS Provision: A History of Processes
Excerpted from Poster Presention at the FDA Science Forum - April 2006
Paulette M. Gaynor, Richard Bonnette, Edmundo Garcia, Jr., Linda S. Kahl, Luis G. Valerio, Jr.
Division of Biotechnology and GRAS Notice Review,
Office of Food Additive Safety (OFAS),
Center for Food Safety and Applied Nutrition, Food and Drug Administration
A GRAS Timeline | Abstract | Excerpts from Legislative History | The GRAS List | Opinion Letters | Comprehensive Review | GRAS Affirmation | GRAS Notification | GRAS Affirmation Petitions | GRAS Notices
A GRAS Timeline
This picture is a graphical representation of a timeline starting in 1906 when the Pure Food and Drug Act was passed. Milestones along the way to the present include the 1938 Federal Food Drug and Cosmetic Act, and the Food Additives Amendment and the GRAS list in 1958. In 1969, President Nixon ordered an evaluation of GRAS substances and in 1972 the GRAS Affirmation process began. The GRAS Notification Program started in 1997 and by the end of 2006, 193 GRAS Notices were filed.
Abstract
Under the 1958 Food Additives Amendment to the Federal Food, Drug, and Cosmetic Act, any substance intentionally added to food is a food additive and is subject to pre-market approval by FDA unless the use of the substance is generally recognized as safe (GRAS; the GRAS provision) (or otherwise excepted from the definition of food additive - e.g., color additive). By 1961, FDA had amended its regulations to include a list of food substances that are GRAS under certain conditions of use ("the GRAS list"). During the 1960's, many manufacturers requested FDA's opinion on whether their conclusions of GRAS status were justified and received "opinion letters." In 1969, FDA removed cyclamate salts from its GRAS list as a result of safety questions, and then-President Nixon directed FDA to reexamine the safety of GRAS substances. In the 1970's, FDA announced that it was conducting a "comprehensive review" of presumed GRAS substances and established rulemaking procedures to affirm the GRAS status of substances that were either on the GRAS list or the subject of a petition ("GRAS affirmation"). To eliminate the resource-intensive rulemaking procedures, in 1997, FDA proposed to replace the GRAS affirmation petition process with a notification procedure ("GRAS notification").
Excerpts from Legislative History
"Proof of the pudding is in the eating" - Origins of Generally Recognized.
Mr. Dies (Texas Congressman): "I think that it [the concept of GRAS] is so vague and indefinite and general that it puts the manufacturer, the processor, in a very bad situation."
"So it seems to me the standard ought to be simplified and eliminate this thing of saying "generally recognized. If you go into the courthouse, who is an expert? How many would have to agree to be generally recognized?"
Mr. Larrick (FDA Commissioner): "Congressmen, all I can say is that we have used this language since 1938 and we have successfully handled over 10,000 new drug applications, and in my opinion the proof of the pudding is the eating."
From Commissioner Larrick's opening remarks
"We believe only those chemicals should be automatically exempted from the new law which are recognized among competent experts as safe for their intended use. This would make it unnecessary, for example, to do studies on table salt, but would not approve the continued use, without proof of safety, of the synthetic emulsifiers now widely used in some fabricated foods."
"The GRAS List"
- 1958 Food Additives Amendment: Congress recognized that many food substances would not require a formal premarket review by FDA to assure their safety, either because:
- Their safety had been established by a long history of use in food; or
- By virtue of the nature of the substances, their conditions of use, and the information generally available to scientists.
- Two-step definition of "food additive:"
- Broadly includes any substance that becomes a component of food or otherwise affects the characteristics of food.
- Excludes substances that are recognized, among qualified experts, as having been adequately shown through scientific procedures (or, in the case of a substance used in food prior to January 1, 1958, through experience based on common use in food) to be safe under the conditions of their intended use.
- December 9, 1958: FDA published a list of GRAS substances and incorporated the list in Title 21 of the Code of Federal Regulations. The current list appears in 21 CFR Parts 182, 184, and 186.
"Opinion Letters"
- Many substances that were considered GRAS by the food industry were not included in FDA's 1958 GRAS list.
- Many manufacturers wrote to FDA and requested an opinion letter in which an FDA official would render an informal opinion on the GRAS status of use of the substance
- Often available only to requestor
- Revoked in 1970 (21 CFR 170.6; 35 FR 5810; April 9, 1970)
"Comprehensive Review"
- October 30, 1969: President Nixon directed FDA to make a critical evaluation of the safety of GRAS food substances.
- March 28, 1972: - Life Sciences Research Office (LSRO) of the Federation of American Societies for Experimental Biology (FASEB) began its contract with FDA to summarize the available scientific literature and to recommend what restrictions, if any, on the use of the substances would be needed to ensure their safe use in food.
- 1970's: LSRO selected qualified scientists (designated as the Select Committee on GRAS Substances (SCOGS)) as consultants to review and evaluate the available information on each of the GRAS substances. The Select Committee's evaluations were made independently of FDA or any other group, governmental or nongovernmental.
- 1970's - 1980's: FDA made tentative reports from SCOGS available to the public and provided opportunity for the public to appear before the Select Committee at a public hearing. SCOGS considered the data, information, and views presented at the hearing in developing its final reports. By 1982, SCOGS had submitted opinions to the FDA on the health aspects of more than 400 substances.
"GRAS Affirmation"
- 1972: FDA conducted rulemaking to establish the procedures (21 CFR 170.35) that it would use to affirm the GRAS status of substances that were the subject of the GRAS review. That rulemaking included a mechanism (the GRAS affirmation petition process) whereby an individual could petition FDA to review the GRAS status of substances not being considered as part of the agency's GRAS review.
- 1970's - 1980's: GRAS Affirmation based on the SCOGS Review
- After receiving a final SCOGS report, FDA reviewed the report and related information
- When appropriate, FDA issued a notice of proposed rulemaking to affirm GRAS status
- If, after reviewing comments to the proposal, FDA concluded that the available data and information supported GRAS status, FDA issued a final rule affirming GRAS status by amending 21 CFR 184 (direct food ingredients) or 186 (indirect food substances).
Examples: Gums, dextrans, various salts (e.g., sodium, potassium, calcium and iron salts),
- 1973 - 1997: GRAS Affirmation Petition Process
- Industry submits GRAS Affirmation Petition
- FDA publishes notice of filing and requests comment
- When appropriate after considering comments, FDA issues a final rule affirming GRAS status
Examples: Canola oil, enzyme preparations, whey, cocoa butter substitute
"GRAS Notification"
- April 17, 1997: FDA proposed to established a a notification procedure whereby a person may inform FDA of a determination that the use of a substance is GRAS (62 FR 18938; April 17, 1997).
- Industry submits GRAS notice
- FDA is evaluating whether each submitted notice provides a sufficient basis for a GRAS determination and whether information in the notice or otherwise available to FDA raises issues that lead the agency to question whether use of the substance is GRAS
- FDA is responding to the notifier by letter
Examples: Phytosterols, DAG oil, enzyme preparations
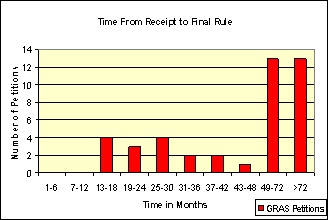
GRAS Affirmation Petitions
Industry sponsored GRAS affirmation petitions completed from 1974 through August 1990. In general, industry sponsored GRAS affirmation petitions completed after that took >72 months. Approximately 20 percent of the substances in 21 CFR Part 184 are the result of industry petitions.
GRAS Notices
GRAS notices completed from 1998 through December 2005. The mean time to respond to these 177 notices is 162 days.