Environmental Impact Statement (EIS) for the FSMA Final Rule for Produce Safety
The following information is dedicated to FDA’s Environmental Impact Statement (EIS) for the FSMA proposed rule entitled Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption (here called the “Produce Safety Proposed Rule” or PS PR). FDA published this proposed rule in the Federal Register on January 16, 2013 (“the 2013 proposed rule”), for codification in 21 CFR Part 112 (78 Fed. Reg. 3504). On March 20, 2013, FDA issued a notice to correct technical errors and errors in reference numbers cited in the 2013 proposed rule (78 Fed. Reg. 17155). Subsequent to the publication of the 2013 proposed rule, extensive information received in public comments led to significant changes in FDA’s thinking, As a result, on September 29, 2014, FDA issued a supplemental notice of proposed rulemaking (“the supplemental proposed rule”), amending certain specific provisions of the 2013 proposed rule (79 Fed. Reg. 58434). Taken together, these publications constitute the PS PR.
This EIS was developed in accordance with the National Environmental Policy Act (NEPA) of 1969, as amended; regulations issued by the Council for Environmental Quality (CEQ), 40 CFR Parts 1500 - 1508; FDA’s Environmental Impact Considerations procedures under 21 CFR Part 25; and other applicable laws. For more information on the development of this EIS, please visit the NEPA Process page of this website.
- Project Overview
- Announcements
- NEPA Process
- FDA Notices
- Public Involvement
- Final Environmental Impact Statement (EIS)
- Record of Decision
- Contact Information
Project Overview
Each year, about 48 million Americans (1 in 6) get sick, 128,000 are hospitalized, and 3,000 die from foodborne diseases, according to estimates from the Centers for Disease Control and Prevention (CDC). Fruits and vegetables are a part of a healthy diet, but fresh produce may have microbial contamination that can cause foodborne illness. FDA’s analysis of available foodborne illness outbreak data from 1996 to 2010 documented 131 outbreaks associated with contaminated produce, causing more than 14,000 serious illnesses.
The FDA Food Safety Modernization Act (FSMA), signed into law by President Obama on Jan. 4, 2011, gives FDA the ability to better protect public health by strengthening the food safety system. The law provides FDA the enforcement authority to prevent food safety problems rather than primarily reacting to problems after they occur. FDA has proposed seven foundational rules for stakeholders along the entire food supply chain to follow, designed to protect public health by promoting safe, sanitary standards to minimize or prevent food safety hazards.
View the History of the Proposed Produce Safety Rule (PDF: 150KB)
FDA prepared a categorical exclusion that was published with the PS PR in January 2013. However, based on currently available information, including comments received from the public, and upon further analysis, FDA determined that an EIS was necessary to assess the environmental (including human) and related socioeconomic impacts of those provisions of the PS PR that may significantly affect the quality of the human environment (here referred to as “potentially significant provisions”), the determination of which was based on public and agency comments prior to and during the EIS scoping period, and alternatives to those provisions.
Announcements
FDA held a scoping meeting that was open to the public on April 4, 2014. This meeting provided the public the opportunity to ask questions about and discuss issues relating to the scope of the EIS. The EIS scoping period ended on April 18, 2014.
On January 12, 2015, FDA made public its Draft EIS for the proposed Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption. On January 14, 2015, FDA published the Notice of Availability (NOA) for the Draft EIS to be published in the Federal Register (80 FR 1852), which began a 60-day public comment period that ended on March 13, 2015.
FDA held a public meeting on the Draft EIS on February 10, 2015. The meeting provided the public the opportunity to ask questions about and discuss issues related to the findings in the Draft EIS.
NEPA Process
This EIS was prepared in accordance with 21 CFR Part 25, Environmental Impact Considerations, and 40 CFR Parts 1500-1508, Council for Environmental Quality (CEQ) Regulations for Implementing NEPA.
On January 4, 2013, FDA released for public comment its proposed Standards for the Growing, Harvesting, Packing, and Holding Produce for Human Consumption. This is one of five proposed rules that would lay the cornerstone of a prevention-based, modern food safety system. FDA published the Produce Safety Proposed Rule (PS PR) in the Federal Register on Jan. 16, 2013, and then published an updated version on March 20, 2013. The published PS PR was accompanied by a categorical exclusion in accordance with FDA’s NEPA procedures at 21 CFR Part 25.30(j).
After reviewing public comments on the PS PR, and reconsidering the potential significant environmental impacts that may have resulted from the its implementation, FDA announced its intent to prepare an EIS and the beginning of the EIS scoping period on Aug.16, 2013. The notice was published in the Federal Register on Aug.19, 2013.
FDA extended the EIS scoping period in both November 2013 and March 2014 to allow the public additional time to review the PS PR and comment on the scope of the EIS, including topics for FDA to consider for evaluation in more detail. FDA held a scoping meeting on April 4, 2014. Participants were provided an opportunity to participate in person at the meeting in College Park, MD, or to connect online through a webinar to listen to the proceedings live. All meeting materials were made available to the public on this website’s project library under the heading of Additional Information and Downloads, and on the FSMA Proposed Rule for Produce Safety website.
Following the EIS scoping period, which ended on April 18, 2013, FDA began preparing a Draft EIS. On September 29, 2014, FDA issued the supplemental proposed rule, amending certain specific provisions of the 2013 proposed rule. The Draft EIS, which is published on this website, takes into consideration comments during the EIS scoping period and comments that were submitted during the comment period for the supplemental proposed rule.
On January 12, 2015, FDA made public its Draft EIS for the proposed Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption. On January 14, 2015, FDA published the Notice of Availability (NOA) for the Draft EIS to be published in the Federal Register, which began a 60-day public comment period that ended on March 10, 2015.
In November 2015, FDA made public its Final EIS for the Proposed Rule and Record of Decision (ROD) for the Final Rule: Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption.
For more information, see the following Constituent Updates:
- FDA to Prepare Environmental Impact Statement on Proposed Produce Rule
- FDA Extends Comment Period on Notice to Determine Scope for the Environmental Impact Statement on Proposed Produce Safety Rule
- FDA Announces Public Meeting, Extension of Comment Period, on Scope of Environmental Impact Statement for Produce Safety Proposed Rule
- Draft Environmental Impact Statement on Proposed Produce Rule Available for Comment
FDA Notices
August 19, 2013, Federal Register Notice: Notice of Intent (NOI) to Prepare an Environmental Impact Statement for the Proposed (Produce Safety) Rule: Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption
November 18, 2013, Federal Register Notice: Notice of Intent (NOI) to Prepare an Environmental Impact Statement for the Proposed (Produce Safety) Rule: Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption, Extension of Comment Period
March 11, 2014, Federal Register Notice: Notice of Intent (NOI) to Prepare an Environmental Impact Statement for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption; Public Meeting on Scoping of Environmental Impact Statement and Extension of Comment Period for Environmental Impact Statement
January 14, 2015, Federal Register Notice: Notice of Availability (NOA) for Draft Environmental Impact Statement for the Proposed Rule: Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption; and notice for Public Meeting on Draft Environmental Impact Statement
November 13, 2015, Federal Register Notice: Notice of Availability (NOA) for Final Environmental Impact Statement and Record of Decision for the Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption
Public Involvement for the Produce Safety Proposed Rule
FDA has participated in an unprecedented level of outreach to producers throughout the United States in the past two years since the publication of the proposed Produce Safety Rule in an effort to hear directly from those who would be most affected by any final rule.
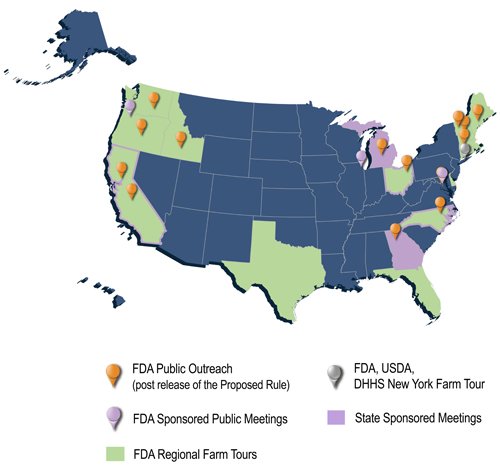
Since the January 2013 release of the proposed Produce Safety Rule, FDA has conducted extensive outreach to all stakeholders who would be impacted by the proposed Produce Safety Rule standards, including more than 100 presentations to industry and consumer groups, farmers, state and local officials, international officials and the research community. Included in this number are three (3) FDA-sponsored public meetings (DC; Chicago; Portland, OR); six (6) sponsored state meetings (NC, GA, CA (2), MI and OH), numerous other listening sessions done via webinar or in-person with stakeholder groups, and meetings in Europe with the EU, WTO and GFSI; and two (2) extensive U.S. regional farm tours in the Pacific Northwest and in New England as well as farm tours in Mexico to discuss the combination of FSMA rules FDA has proposed this year to help ensure the safety of both domestic and imported foods. Outreach efforts by the Office of Foods and Veterinary Medicine and program headquarters staff have been complemented by the outreach of our field and foreign offices who have also been actively conducting outreach in the various regions where we have postings.
Prior to the release of the Proposed Produce Safety Rule, senior FDA staff visited more than 20 farms in 13 states and interfaced with hundreds of stakeholders at various meetings across the country to develop the Proposed Produce Safety Rule. Many of these meetings included senior officials from the U.S. Department of Agriculture, as well as state commissioners of agriculture.
Map of FDA's Public Involvement
Download the Produce Safety Rule Public Outreach Poster (PDF - 2MB).
Final Environmental Impact Statement (EIS)
The EIS assesses the environmental (including human) and related socioeconomic impacts for the potentially significant provisions and alternatives to those provisions. It also assesses the No Action Alternative, which is made up of baseline agricultural practices, regulations, and industry programs, as well as background environmental conditions. In the Final EIS, FDA identifies the “Agency’s preferred alternative,” i.e., the alternative which the Agency believes, when the Produce Safety proposed rule is finalized, would fulfill its statutory mission and responsibilities, giving consideration to economic, environmental, technical, and other factors.
Download the Final Environmental Impact Statement (PDF - 15.5MB)
For more information on the final statement, see:
-
Docket No. FDA-2014-N-2244, includes submitted comments
Record of Decision (ROD)
The ROD identifies both the environmentally preferable alternative and the agency’s preferred alternative. It also identifies the alternatives considered and explains the agency’s rationale for selecting the preferred alternative.
Download the Record of Decision (PDF - 370KB)
For more information on the final statement, see:
-
Docket No. FDA-2014-N-2244, includes submitted comments
Contact Information
For general information about the FDA NEPA process, or about this Environmental Impact Statement (EIS), please contact:
Annette McCarthy, Ph.D.
Center for Food Safety and Applied Nutrition (HFS-205)
Food and Drug Administration
5100 Paint Branch Pkwy.
College Park, MD 20740
Phone: (240) 402-1057