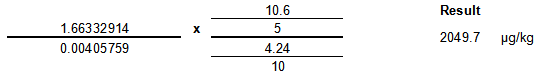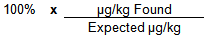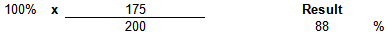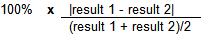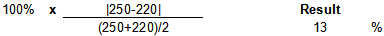Laboratory Information Bulletin (LIB) 4422: Melamine and Cyanuric Acid Residues In Foods
October 2008
Interim Method for Determination of Melamine and Cyanuric Acid Residues In Foods using LC-MS/MS: Version 1.0
Michael Smoker1 and Alexander J. Krynitsky2
1 Kansas City Laboratory, Office of Regulatory Affairs, Food and Drug Administration
2 Office of Regulatory Science, Center for Food Safety and Applied Nutrition, Food and Drug Administration
Abstract
This procedure has been validated for the determination of melamine and cyanuric acid in both pork and fish tissue using liquid chromatography with triple quadrupole tandem mass spectrometry (LC-MS/MS) The method has only been evaluated for performance, but not fully validated, in infant formula. In this procedure, both cyanuric acid and melamine are extracted from tissue and infant formula with a 50-50 acetonitrile-water extraction solution, followed by centrifugation. The cleanup procedures for melamine involve mix mode cation exchange (MCX) solid phase extraction (SPE) and that for cyanuric acid uses mix mode anion exchange (MAX) SPE. Consequently, aliquots of the same extract are individually processed with the two modes of SPE. The final cleaned up extracts for both melamine and cyanuric acid are in acetonitrile, making the procedure amenable to evaporate the excess solvent for sensitivity needs or solvent exchange (depending on the LC column used). Each compound is analyzed separately using a zwitterionic HILIC LC column. Electrospray ionization is used in both the negative ion (cyanuric acid) and positive ion (melamine) modes. Two selected reaction monitoring (SRM) transitions are monitored for both compounds. Commercially available, isotopically labeled internal standards for each compound were used to correct for any matrix effects. The method limit of quantitation (LOQ) for melamine was: 25 µg/kg for tissue and liquid formula and 200 µg/kg for dryinfant formula powder. The method LOQ for cyanuric acid was: 50 µg/kg for tissue and liquid formula and 200 µg/kg for dry infant formula powder. Fortified test portions were within 75-125% recovery. Determination of incurred residue in tissue agreed well with the results of an independent laboratory.
This method for Melamine and Cyanuric Acid should be regarded as interim. Because of the need to rapidly provide this information, the method has not undergone the rigorous internal and external validation required for an official method. The performance of the method may change when different equipment and supplies are used or when different sample matrices are encountered. The user should validate the performance of the method in their laboratory and pay particular attention to the recommended quality control elements. Users should document with their results the version or date of the method used.
Introduction
In 2007, pet food, animal feed, wheat gluten, and other protein-based food commodities were found to contain residues of melamine and a related compound, cyanuric acid. Widespread pet illness and death was subsequently attributed to the formation of melamine-cyanurate crystals in the kidneys of these animals. At that time, several laboratories developed methods for these compounds in pet food, raw protein sources, and other animal feeds. Because animals may eat food contaminated with melamine residues, analytical methods to determine melamine residues in fish and animal tissues were also developed. Recently, there has been a problem with contamination of infant formula with melamine and cyanuric acid in China. This is suspected of causing illness to many children in that country.
A procedure has been validated for the determination and identification of melamine and cyanuric acid in animal tissue and the same procedure has been evaluated for the determination and identification of the above in milk-based infant formula.
Summary of Procedure
This procedure involves the determination of melamine and cyanuric acid by LC-MS/MS in animal tissue and infant formula using isotopically labeled internal standards for each compound in order to correct for matrix effects. Prior to the extraction procedure, 250 ng of isotopically labeled melamine is added to the test portion, followed by the addition of 2500 ng of isotopically labeled cyanuric acid. Tissue samples and liquid infant formula (5 g) are extracted with 20 mL 50-50 acetonitrile-water in a 50 mL polypropylene centrifuge tube. For a 1 g sample of dry infant formula, 4 mL of de-ionized water is first added followed by 20 mL of 50-50 acetonitrile-water. Extracts are centrifuged at 3750 g and then cleaned up using solid phase extraction. The cleanup procedure for melamine involves MCX SPE and that for cyanuric acid uses MAX SPE. Consequently, aliquots of the same extract are individually processed with the two modes of SPE. The final extracts for both melamine and cyanuric acid are in acetonitrile, making the procedure amenable to evaporate the excess solvent for sensitivity needs or solvent exchange (depending on the LC column used). Each compound is analyzed separately using a zwitterionic HILIC LC column. Electrospray ionization is used in both the negative ion (cyanuric acid) and positive ion (melamine) modes. Two selected reaction monitoring (SRM) transitions are monitored for both compounds.
1. Standards
- Calibration and spiking concentrations can be varied as needed
- Internal standard levels can also be adjusted as dictated by instrument sensitivity and availability of labeled material
- Melamine
- Isotopically labeled Melamine (Cambridge Isotope Labs. #CNLM-8150-1.2) 13C315N3 labeled Melamine
- Prepare isotopically labeled Melamine stock solution at 1 μg/mL in acetonitrile
- Native Melamine (Aldrich #240818-5G)
- Stock and Spiking Solutions
- Prepare 10 μg/mL solution in 50:50 acetontirile:water
- Stock and Spiking Solutions
- Calibration Curve
- Prepare solutions of 0, 1, 10, 50, 250, and 1000 ng/mL of Native Melamine in 2% diethylamine/acetonitrile
- Prepare each calibration solution with isotopically labeled Melamine at 10 ng/mL
- Cyanuric Acid
- Isotopically labeled Cyanuric Acid (Cambridge Isotope Labs. #CNLM-4661-1.2) 13C315N3 labeled Cyanuric Acid
- Alternate Source for Isotopically Labeled Cyanuric Acid: Isotec; 13C3 Cyanuric Acid.
- Prepare isotopically labeled Cyanuric Acid stock solution at 5 μg/mL solution in acetonitrile
- Native Cyanuric Acid (TCI #C0459)
- Stock and Spiking
- Prepare a 10 μg/mL solution in 50:50 acetonitrile:water
- Stock and Spiking
- Calibration Curve
- Prepare solutions of 0, 10, 50, 200, 500, and 1000 ng/mL Native Cyanuric Acid in 4% formic acid in acetonitrile
- Prepare each calibration solution with isotopically labeled Cyanuric Acid at 100 ng/mL
2. Reagents
- Acetonitrile UV grade (Burdick & Jackson #015-4)
- Formic Acid 98+% (Riedel de Haën #33015)
- Diethylamine (DEA) (Sigma-Aldrich #D0806-1L)
- Ammonium Hydroxide, concentrated, redistilled (GFS Chemicals #810)
- Water (De-Ionized)
- Ammonium Acetate, 99.99+% (Aldrich #431311-250G)
3. Other Consumables
- 50 mL BD Falcon Bluemax polypropylene graduated tubes, (BD Biosciences #352098)
- 15 mL BD Falcon Bluemax Jr. polypropylene graduated tubes (BD Biosciences #352097)
- 3 mL Norm-Ject AirTite Leur-lock disposable syringes (AirTite Normject #AL3)
- Nalgene Non-Sterile PTFE Syringe filters, 0.2 micron, 25 mm (Nalgene #199-2020)
- Waters Oasis MCX SPE Cartridges, 6cc 150mg 60 micron (Waters #18600255)
- Waters Oasis MAX SPE Cartridges, 6cc 150mg 60 micron (Waters #18600370)
- SeQuant ZIC HILIC column, 2.1x150mm PEEK, 5 micron (The Nest Group, #Q2712-152)
- Autosampler vials (Waters #WAT094219)
- Autosampler caps with pre-slit septa (Waters #186002648)
4. Equipment
- Standard 24-port SPE manifold (Supelco #57250-U)
- Blending Apparatus (Brinkmann Instruments Polytron PT 10/35)
- Wrist-action shaker (Burrell Scientific Model 75)
- ThermoFinnigan Surveyor HPLC System (Thermo Fisher Scientific)
- ThermoFinnigan TSQ Quantum Mass Spectrometer (Thermo Fisher Scientific)
- Xcalibur Software (Thermo Fisher Scientific)
5. Sample Preparation/Extraction
- Weigh 5 grams of sample (tissue or liquid infant formula) into 50 mL Falcon Tube, record weight in notebook
- For samples with a low water content (e.g. powdered infant formula), weigh 1 gram of sample and add 4 mL of water
- To all add 250 ng (250 µl of 1 µg/ml stock) of isotopically labeled Melamine
- To all add 2500 ng (250 µl of 10 µg/ml stock) of isotopically labled Cyanuric Acid
- To the spikes, add desired amount of native Melamine and the desired amount of native Cyanuric Acid
- Add 20 mL of extraction solution (50-50 ACN-Water)
- Blend with Polytron until sample is smooth (about 10-20 seconds)
- For liquid or highly soluble samples, the blending step can be skipped
- Shake 10-20 min
- Centrifuge to separate layers
- For liquid or highly soluble samples, centrifugation may be skipped
6. Melamine SPE Cleanup
- Condition SPE tubes and load sample
- Place the MCX SPE tubes on the manifold
- Add about 5 mL of acetonitrile and allow to gravity drip
- Add about 5 mL of 4% formic acid in WATER and allow to gravity drip
- Close valves to tubes
- Add 3 mL of 4% formic acid in WATER
- Add 2 mL of sample extract solution (if layers are present in the extract solution, take 2 mL from clear or supernatant layer if possible)
- Open valves, allow to gravity drip
- Add about 5 mL acetonitrile and allow to gravity drip
- Add about 5 mL of 0.20% DEA in acetonitrile and allow to gravity drip
- Briefly apply light vacuum for approximately 10 seconds in order to dry SPE cartridge.
- Collect sample
- Label a 15 mL Falcon Tube for each sample to collect final eluent.
- Set up labeled Falcon Tubes under the corresponding MCX SPE tubes
- Gravity elute sample into Falcon Tubes with 4 mL of 2 % DEA in acetonitrile
- Once SPE tube appears empty, apply light vacuum to obtain all remaining eluent
- Mix sample eluent
- Filter 1 mL of eluent into autosampler vial using syringe filters and syringes
7. Cyanuric Acid SPE Cleanup
- Condition SPE tubes and load sample
- Place the MAX SPE tubes on the manifold
- Add about 5 mL of acetonitrile and allow to gravity drip
- Add about 5 mL of 5% ammonium hydroxide in water and allow to gravity drip
- Close valves to tubes
- Add 3 mL of 5% ammonium hydroxide in water
- Add 2 mL of sample extract solution (if layers are present in the extract solution, take 2 mL from clear or supernatant layer if possible)
- Open valves, allow to gravity drip
- Add about 5 mL acetonitrile and allow to gravity drip
- Briefly apply light vacuum for approximately 10 seconds in order to dry SPE cartridge.
- Collect sample
- Label a 15 mL Falcon Tube for each sample to collect final eluent
- Set up labeled Falcon Tubes under the corresponding MAX SPE tubes
- Gravity elute sample into Falcon Tubes with 2 mL of 4% formic acid in ACETONITRILE
- Once SPE tube appears empty, apply light vacuum to obtain all remaining eluent
- Mix sample eluent
- Filter 1 mL of eluent into autosampler vial using syringe filters and syringes
8. HPLC Conditions
- Mobile Phase A: 5% 10mM ammonium acetate : 95% acetonitrile
- For example, 50 mL of 10 mM ammonium acetate mixed with 950 mL of acetonitrile
- Mobile Phase B: 10mM ammonium acetate dissolved in 50:50 acetonitrile:water
- Expected Retention Times
- Melamine 7-12 min
- Cyanuric Acid 5-10 min
- Syringe Flush/Wash Solution: 50:50 methanol:water
- Pump Program
- 1 - 10 minutes: 100% A at 0.3 mL/min
- 10.1 - 15 minutes: 50% A and 50% B at 0.6 mL/min
- 15.1 - 20 minutes: 100% A at 0.6 mL/min
- 20.1 - 30 minutes: 100% A at 0.3 mL/min
- 20 microliter full-loop injection at 8 microliter/min
- 250 microliter syringe flush at 50 microliter/min
- 250 microliter syringe wash
Caution: The melamine fractions are analyzed separately from the cyanuric acid fractions in order to guard against the formation of the melamine-cyanurate complex.
9. Mass Spectrometer Settings
- Tune the instrument's parameters to optimize response for each compound
- Cyanuric Acid
- Negative ESI
- Spray Voltage -3500 V
- Sheath Gas 30
- Aux Gas 15
- Capillary Temp 300 C
- Internal standard transition: m/z 134→44 (based on 13C315N3 labeled cyanuric acid); Collision Energy 12 eV, Scan Time 0.2s
- this transition must be adjusted depending on the degree of isotope subsitution in the labeled material. Important!
- the transition will be m/z 131→43 if the alternative source of labeled cyanuric acid is used (e.g. 13C3 labeled cyanuric acid)
- this transition must be adjusted depending on the degree of isotope subsitution in the labeled material. Important!
- Native Primary transition: m/z 128→42; Collision Energy 12 eV, Scan Time 0.2s
- Native Secondary transition: m/z 128→85; Collision Energy 17 eV, Scan Time 0.2s
- Negative ESI
- Melamine
- Positive ESI
- Spray Voltage +4000V
- Sheath Gas 15
- Aux Gas 8
- Capillary Temp 300 C
- Internal standard transition: m/z 133→89 (based on 13C315N3 labeled melamine); Collision Energy 25 eV, Scan Time 0.2s
- this transition must be adjusted depending on the degree of isotope subsitution in the labeled material. Important!
- Internal standard transition will be m/z 130→87 if alternative internal standard is used (e.g. 13C3 labeled melamine)
- this transition must be adjusted depending on the degree of isotope subsitution in the labeled material. Important!
- Native Primary transition: m/z 127→85; Collision Energy 25 eV, Scan Time 0.2s
- Native Secondary transition: m/z 127→68; Collision Energy 25 eV, Scan Time 0.2s
- Positive ESI
Identification Criteria: The individual selective reaction monitoring (SRM) ion ratios in the test portion extracts should be within ±25% with respect to the calibration standards.
Limit of Quantitation (LOQ): The analytical solution quantitation limit (ASQL) is defined as the level at which a 10:1 peak to peak signal/noise ratio is observed for the analyte quantitation ion transition (primary transition) and 3:1 peak to peak signal/noise ratio observed for the analyte secondary ion transition. The limit of quantitation (LOQ) is the ASQL adjusted for the analytical portion's mass and dilution.
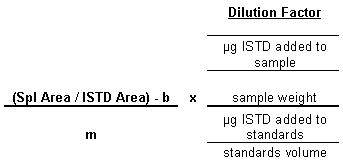
10. Calculations
Internal Standard Calculation
Example Calculation
| Item | Value | Units |
|---|---|---|
| Sample Area | 94664 | NA |
| Internal Standard Area | 57343 | NA |
| b (intercept) | -0.0124912 | NA |
| m (slope) | 0.00405759 | ng/mL |
| μg ISTD added to sample | 10.6 | μg |
| sample weight | 5 | g |
| μg ISTD added to standards | 4.24 | μg |
| standards volume | 10 | mL |
Spike Recovery Calculation
Example Calculation
| µg/kg Found | 175 | µg/kg | |
| Expected µg/kg | 200 | µg/kg | |
Spike Relative Percent Difference (RPD) Calculation
Example Calculation
| result 1 | 250 | µg/kg | |
|---|---|---|---|
| result 2 | 220 | µg/kg | |
11. Summary of Validation Results for Tissue
| Sample | Quan 127→85 | Conf 127→68 | ||
|---|---|---|---|---|
| µg/kg Found | Recovery | µg/kg Found | Recovery | |
| Reagent Blank* | 3.4 | - | 3.5 | - |
| Duplicate Reagent Blank* | 3.0 | - | 3.2 | - |
| Control | 3.7 | - | 4.7 | - |
| Duplicate Control | 3.8 | - | 4.6 | - |
| 25 µg/kg spike 1 | 29.0 | 116 | 31.0 | 124 |
| 25 µg/kg spike 2 | 26.7 | 107 | 26.0 | 104 |
| 25 µg/kg spike 3 | 27.6 | 111 | 30.5 | 122 |
| 25 µg/kg spike 4 | 27.9 | 112 | 28.6 | 114 |
| 25 µg/kg spike 5 | 30.1 | 120 | 28.1 | 113 |
| 25 µg/kg spike 6 | 26.3 | 105 | 26.4 | 105 |
| 200 µg/kg spike | 189.1 | 95 | 190.1 | 95 |
| 200 µg/kg spike duplicate | 192.4 | 96 | 193.4 | 97 |
| 1000 µg/kg spike | 934.6 | 93 | 934.7 | 93 |
| 1000 µg/kg spike duplicate | 934.2 | 93 | 926.3 | 93 |
| Sample | Quan 128→42 | Conf 128→85 | ||
|---|---|---|---|---|
| µg/kg Found | Recovery | µg/kg Found | Recovery | |
| Reagent Blank | - | - | - | - |
| Duplicate Reagent Blank | - | - | - | - |
| Control | - | - | - | - |
| Duplicate Control | - | - | - | - |
| 50 µg/kg spike 1 | 59.0 | 118 | 60.5 | 121 |
| 50 µg/kg spike 2 | 57.4 | 115 | 58.4 | 117 |
| 50 µg/kg spike 3 | 58.2 | 116 | 64.3 | 129 |
| 50 µg/kg spike 4 | 58.7 | 117 | 63.0 | 126 |
| 50 µg/kg spike 5 | 57.5 | 115 | 60.7 | 121 |
| 50 µg/kg spike 6 | 60.4 | 121 | 58.2 | 116 |
| 200 µg/kg spike | 205.9 | 103 | 200.4 | 100 |
| 200 µg/kg spike duplicate | 201.7 | 101 | 197.7 | 99 |
| 1000 µg/kg spike | 970.6 | 97 | 958.5 | 96 |
| 1000 µg/kg spike duplicate | 969.1 | 97 | 957.6 | 96 |
| Sample | Quan 127→85 | Conf 127→68 | ||
|---|---|---|---|---|
| µg/kg Found | Recovery | µg/kg Found | Recovery | |
| Reagent Blank* | 3.6 | - | 3.6 | - |
| Control | 3.1 | - | 5.0 | - |
| 200 µg/kg spike | 209.5 | 105 | 208.5 | 104 |
| 200 µg/kg spike duplicate | 208.2 | 104 | 198.0 | 99 |
| Sample | Quan 128→42 | Conf 128→85 | ||
|---|---|---|---|---|
| µg/kg Found | Recovery | µg/kg Found | Recovery | |
| Reagent Blank | - | - | - | - |
| Control | - | - | - | - |
| 200 µg/kg spike | 174.3 | 87 | 157 | 79 |
| 200 µg/kg spike duplicate | 174.0 | 87 | 159 | 79 |
| Sample | Quan 127→85 | Conf 127→68 | ||
|---|---|---|---|---|
| µg/kg Found | Recovery | µg/kg Found | Recovery | |
| Reagent Blank* | 5.3 | - | 5.3 | - |
| Control | 11.8 | - | 11.8 | - |
| 200 µg/kg spike | 199.7 | 100 | 203.2 | 102 |
| 200 µg/kg spike duplicate | 212.3 | 106 | 229.1 | 115 |
| Sample | Quan 128→42 | Conf 128→85 | ||
|---|---|---|---|---|
| µg/kg Found | Recovery | µg/kg Found | Recovery | |
| Reagent Blank | - | - | - | - |
| Control | - | - | - | - |
| 200 µg/kg spike | 192.8 | 96 | 159.5 | 80 |
| 200 µg/kg spike duplicate | 192.5 | 96 | 171.0 | 85 |
| Sample | Melamine | Cyanuric Acid | ||
|---|---|---|---|---|
| CFSAN result, mg/kg | KAN-LAB result, mg/kg | CFSAN result, mg/kg | KAN-LAB result, mg/kg | |
| FCC Catfish | 0.057 | 0.056 | 0.015 | NF |
| Pig Control | 0.02 | 0.008 | <> | NF |
| Pig fed Cyanuric Acid 1 | 0.285 | 0.292 | 1.8 | 2.05 |
| Pig fed Cyanuric Acid 2 | 0.265 | 0.287 | 1.78 | 2.05 |
| Pig fed Melamine 1 | 49.1 | 47.7 | 0.043 | 0.043 |
| Pig fed Melamine 2 | 47 | 50 | 0.044 | 0.042 |
| Pig fed Melamine and Cyanuric Acid 1 | 57.4 | 60.1 | 1.91 | 2.31 |
| Pig fed Melamine and Cyanuric Acid 2 | 58.1 | 61.2 | 1.97 | 2.36 |
| Carryover Check | Peak Area | % |
|---|---|---|
| Melamine 1000 µg/kg Standard | 44029816 | - |
| Subsequent Blank Injection | 1603 | 0.004 |
| Melamine 1000 µg/kg Spike | 4438114 | - |
| Subsequent Blank Injection | 3510 | 0.079 |
| Cyanuric Acid 1000 µg/kg Standard | 381798 | - |
| Subsequent Blank Injection | NF | 0.000 |
| Cyanuric Acid 1000 µg/kg Spike | 96574 | - |
| Subsequent Blank Injection | NF | 0.000 |
12. Results of Method Performance in Infant Formula
Sample: Milk-based ready-to-feed infant formula (Liquid)
Preparation: Extracted 5 grams of liquid by shaking with 20 mL of 50:50 ACN:Water
| Sample | Quan 127→85 | Conf 127→68 | ||
|---|---|---|---|---|
| µg/kg Found | Recovery | µg/kg Found | Recovery | |
| Reagent Blank | 3.0 | - | 3.7 | - |
| Control | 3.1 | - | 3.9 | - |
| 10 µg/kg spike* | 12.2 | 92 | 13.0 | 93 |
| 10 µg/kg spike duplicate* | 13.2 | 102 | 13.1 | 94 |
| Reagent Blank | 2.9 | - | 3.6 | - |
| Control | 3.7 | - | 3.8 | - |
| 100 µg/kg spike* | 104.2 | 101 | 101.2 | 98 |
| 100 µg/kg spike duplicate* | 106.3 | 103 | 102.9 | 99 |
| Sample | Quan 128→42 | Conf 128→85 | ||
|---|---|---|---|---|
| µg/kg Found | Recovery | µg/kg Found | Recovery | |
| Reagent Blank | - | - | - | - |
| Control | - | - | - | - |
| 100 µg/kg spike | 85.2 | 85 | 85.6 | 86 |
| 100 µg/kg spike duplicate | 70.8 | 71 | 53.0 | 53 |
| Reagent Blank | - | - | - | |
| Control | - | - | - | |
| 500 µg/kg spike | 464.9 | 93 | 444.8 | 89 |
| 500 µg/kg spike duplicate | 435.6 | 87 | 432.1 | 86 |
13. Dry Infant Formula Performance Results When Analyzing Samples from Field Assignment
Sample: Various Matrices (see description)
Preparation: Per Method
§ Indicates what appears to be BACKGROUND MELAMINE (peaks are confirmed)
NF = Not Found (< 50µg/kg for dry product)
| Sample | Batch | Description | Quan 127→85 | Conf 127→68 | ||||
|---|---|---|---|---|---|---|---|---|
| µg/kg Found | %Rec | µg/kg Found | %Rec | |||||
| Reagent Blank | 1 | - | 36.3 § | - | 43.1 § | - | ||
| Sample A Spike 1 500 µg/kg | 1 | inf formula powder | 434.4 | 87 | 435.0 | 87 | ||
| Sample A Spike 2 500 µg/kg | 1 | inf formula powder | 446.7 | 89 | 438.9 | 88 | ||
| Reagent Blank | 2 | - | 42.4 § | - | 43.1 § | - | ||
| Sample H Spike 1 500 µg/kg | 2 | inf formula powder | 507.7 | 102 | 513.1 | 103 | ||
| Sample H Spike 2 500 µg/kg | 2 | inf formula powder | 492.9 | 99 | 502.4 | 100 | ||
| Reagent Blank | 3 | - | 40.8 § | - | 42.2 § | - | ||
| Sample P Spike 1 500 µg/kg | 3 | inf formula powder | 483.5 | 97 | 493.2 | 99 | ||
| Sample P Spike 2 500 µg/kg | 3 | inf formula powder | 486.4 | 97 | 490.3 | 98 | ||
| Sample | Batch | Description | Quan 128→42 | Conf 128→85 | ||||
|---|---|---|---|---|---|---|---|---|
| µg/kg Found | %Rec | µg/kg Found | %Rec | |||||
| Reagent Blank* | 1 | - | NF | - | NF | - | ||
| Sample A Spike 1 500 µg/kg | 1 | inf formula powder | 475.8 | 95 | 403.6 | 81 | ||
| Sample A Spike 2 500 µg/kg | 1 | inf formula powder | 503.0 | 101 | 442.0 | 88 | ||
| Reagent Blank | 2 | - | NF | - | NF | - | ||
| Sample H Spike 1 500 µg/kg | 2 | inf formula powder | 397.7 | 80 | 329.6 | 66 | ||
| Sample H Spike 2 500 µg/kg | 2 | inf formula powder | 471.1 | 94 | 357.5 | 71 | ||
| Reagent Blank | 3 | - | NF | - | NF | - | ||
| Sample P Spike 1 2500 µg/kg | 3 | inf formula powder | 2483.4 | 99 | 2416.0 | 97 | ||
| Sample P Spike 2 2500 µg/kg | 3 | inf formula powder | 2544.4 | 102 | 2407.6 | 96 | ||
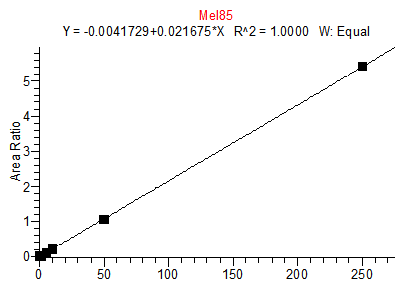
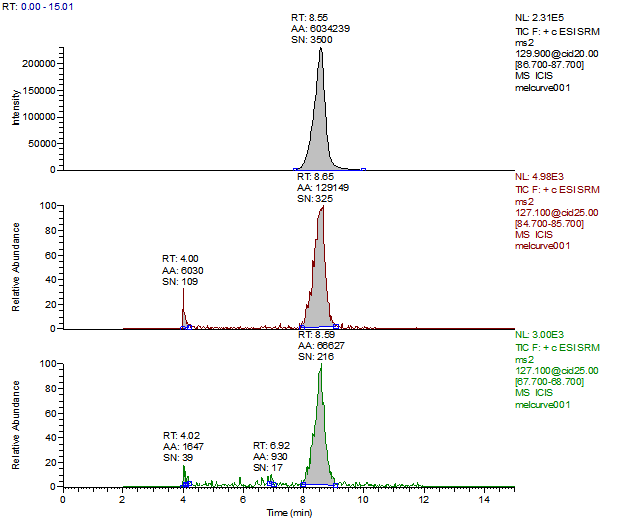
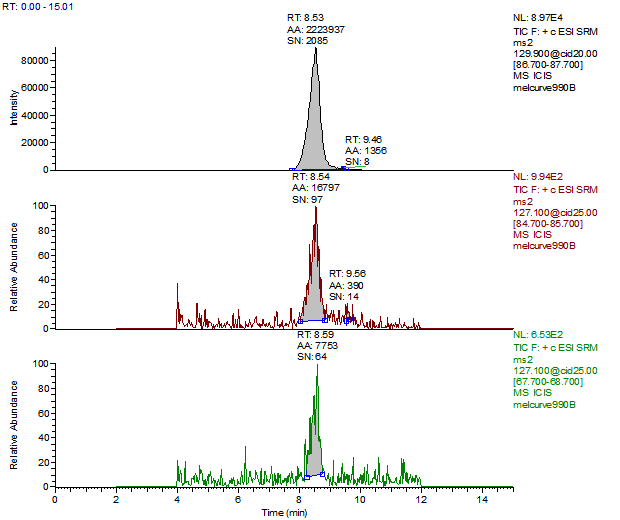
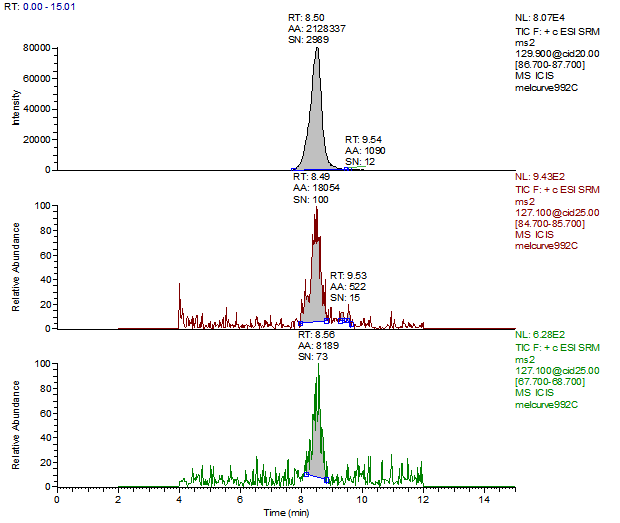
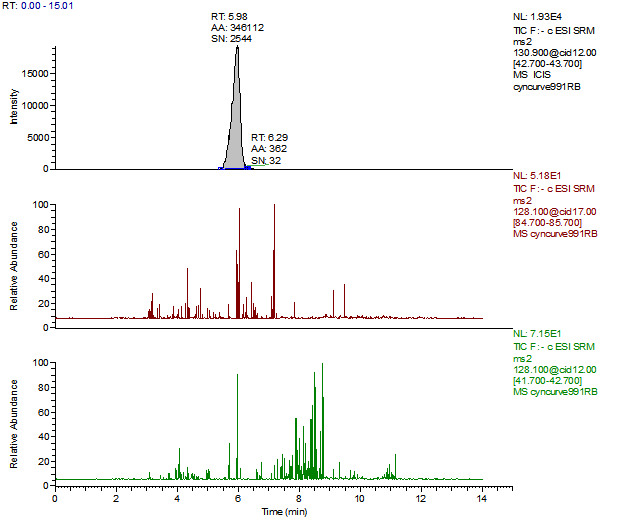
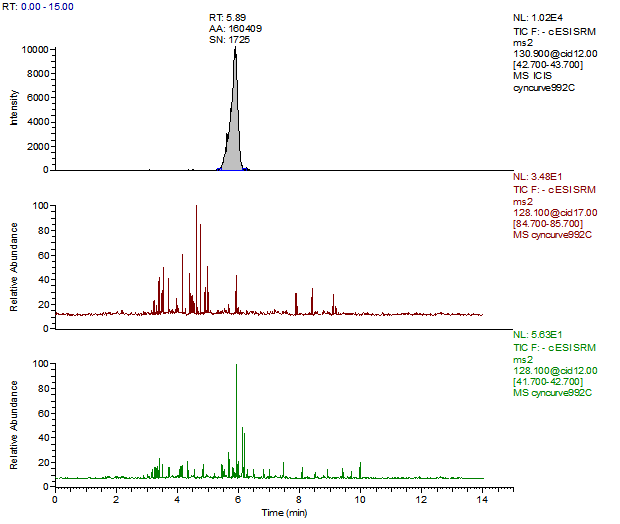
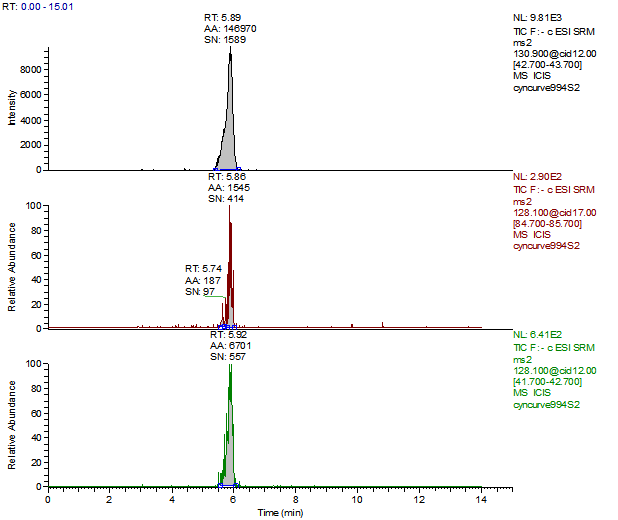
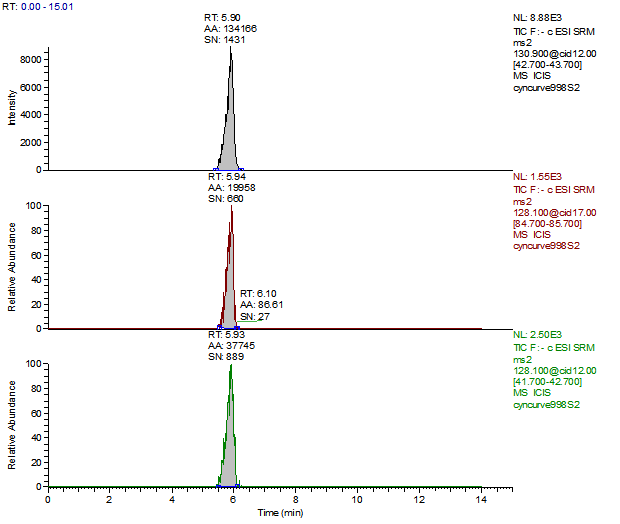
14. Example Chromatograms and Calibration Curves
Melamine Calibration Standards
0, 1, 5, 10, 50 and 250 ng/mL with 13C3 ISTD @ 12 ng/mL
(Prepared in 2% DEA in ACN)
Melamine 1ng/mL Standard
Melamine Reagent Blank
Melamine Control Sample (Liquid Infant Formula)
Melamine 10 µg/kg Spike (Liquid Infant Formula)
Melamine 100 µg/kg Spike (Liquid Infant Formula)
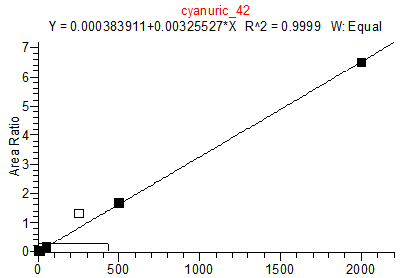
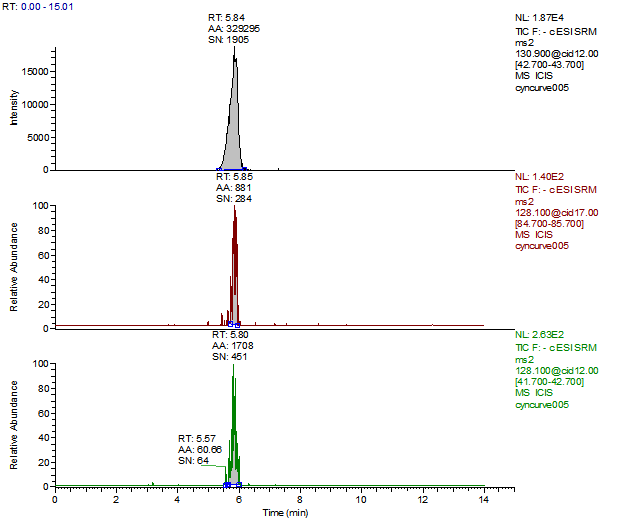
Cyanuric Acid Calibration Standards
0, 5, 10, 50, 250, 500 and 2000 ng/mL with 13C3 ISTD @ 424 ng/mL
(Prepared in 4% Formic Acid in ACN)
* 250 ng/mL point has been excluded
Cyanuric Acid 5 ng/mL Standard
Cyanuric Acid Reagent Blank
Cyanuric Acid Control
Cyanuric Acid 100 µg/kg Spike (Liquid Infant Formula)
Cyanuric Acid 500 µg/kg Spike (Liquid Infant Formula)
Results and Discussions
A fast, sensitive, and specific method was developed for the determination of melamine and cyanuric acid in foods. Even though the original method was developed and validated for tissue, it appears to be adequately performing for the routine analysis of the above compounds in dry products such as infant formula. The use of the isotopically labeled standards is of importance in order to correct for any matrix effects or volume changes. Signal suppression averaged 50% for melamine in tissue samples and infant formula, while for cyanuric acid; the signal suppression averaged 40% for the same matrices tested. The individual selective reaction monitoring (SRM) ion ratios in the test portion extracts were within ±10% with respect to the calibration standards. This was well within our criteria of ±25%. The method limit of quantitation (LOQ) for melamine was: 25 µg/kg for tissue and liquid formula and 200 µg/kg for dry infant formula products. The reason for such a high LOQ for melamine in the dry infant formula is that one laboratory was experiencing high background levels for melamine in reagent blanks, when analyzing dry products. These reagent background levels, when calculated as equivalent to a 1.0 gram dry weight test portion, average 40 µg/kg. It was therefore necessary to increase the LOQ for melamine in dry infant formula products by five fold. This phenomenon was not observed with cyanuric acid. However, since cyanuric acid is traditionally less sensitive than melamine, 200 µg µg/kg /kg was found to be the LOQ for cyanuric acid in dry infant formula products. Fortified test portions were within 75-125% recovery. Determination of incurred residue in tissue agreed well with the results of an independent laboratory. At this time we have not been combining the melamine fraction with the cyanuric acid fraction since we do not know whether it will form the insoluble cyanuric acid complex. Further work on this topic is being investigated along with application and validation of the method for additional food matrices.
Acknowledgment
Authors wish to thank FDA Center for Veterinary Medicine for pork tissue containing incurred residues of melamine and cyanuric acid and FDA Forensic Chemistry Center for catfish containing incurred residues melamine and cyanuric acid. The authors also wish to thank Gonçalo Gamboa, FDA National Center for Toxicological Research, for initially providing labeled standards for both melamine and cyanuric acid before they became commercially available. Finally, the authors wish to thank Gregory Diachenko, Sherri Turnipseed, John Callahan, Douglas Heitkemper, and Narong Chamkasem for helpful discussions.
References
- Heller, DN, Nochetto, C. Simultaneous Determination and Confirmation of Melamine and Cyanuric Acid in Animal Feed by Zwitterionic HILIC Chromatography and Tandem Mass Spectrometry, in press, Rapid Commun. Mass Spectrom.
- Litzau J, Mercer G, Mulligan K. http://www.fda.gov/cvm/GCMSMelamine.htm. Accessed February 25, 2007.
- Andersen WC, Turnipseed SB, Karbiwnyk CM, Madson MR. U.S. FDA Laboratory Information Bulletin, 2007: 23: 4396. LIB No. 4396 accessed February 28, 2008.
- Karbiwnyk, CK, Andersen, WC, Turnipseed, SB, Storey, JM, Madson, MR, Miller, KE, Gieseker, CM, Miller, RA, Rummel, NG, Reimschuessel, R. Determination of cyanuric acid residues in catfish, trout, tilapia, salmon and shrimp by LC- MS/MS, in press, Anal. Chim Acta
- Andersen WC, Turnipseed SB, Karbiwnyk CM, Clark, SB, Madson MR, Gieseker CM, Miller RA, Rummel NG, Reimschuessel R. Determination and Confirmation of Melamine Residues in Catfish, Trout, Tilapia, Salmon and Shrimp by LC-MS-MS. 2008, J. Ag. Food Chem 56: 4340.
- Reimschuessel R., Gieseker, CM, Miller, RA, Ward, J, Boehmer, J, Rummel, N, Heller, DN, Nochetto, CN, de Alwis, H, Bataller, N, Andersen, WC, Turnipseed, SB, Karbiwnyk, CM, Satzger, D, Crowe, JB, Wilber, NR, Reinhard, MK, Roberts, JF, Witkowski, MR Evaluation of the renal effects of experimental feeding of melamine and cyanuric acid to fish and pigs. 2008, Am. J. Vet. Res., 69:1217.
- Dobson RLM, Motlagh S, Quijano M, Cambron RT, Baker TR, Pullen AM, Regg BT, Bigalow-Kern AS, Vennard T, Fix A, Reimschuessel R, Overmann G, Shan Y, Dasont GP. Toxicol Sci, ePub Aug 9, 2008.
Chemical Name: melamine
IUPAC International Chemical Identifier: InChI=1/C3H6N6/c4-1-7-2(5)9-3(6)8-1/h(H6,4,5,6,7,8,9)
Chemical Name: cyanuric acid
IUPAC International Chemical Identifier: InChI=1/C3H3N3O3/c7-1-4-2(8)6-3(9)5-1/h(H3,4,5,6,7,8,9)