CDER Data Standards Program
What’s New
December 2025
- CDER-CBER Data Standards Program Joint Action Plan (PDF - 301 KB)
July 2025
- CBER-CDER Data Standards Program 2024 Annual Assessment (PDF - 344 KB)
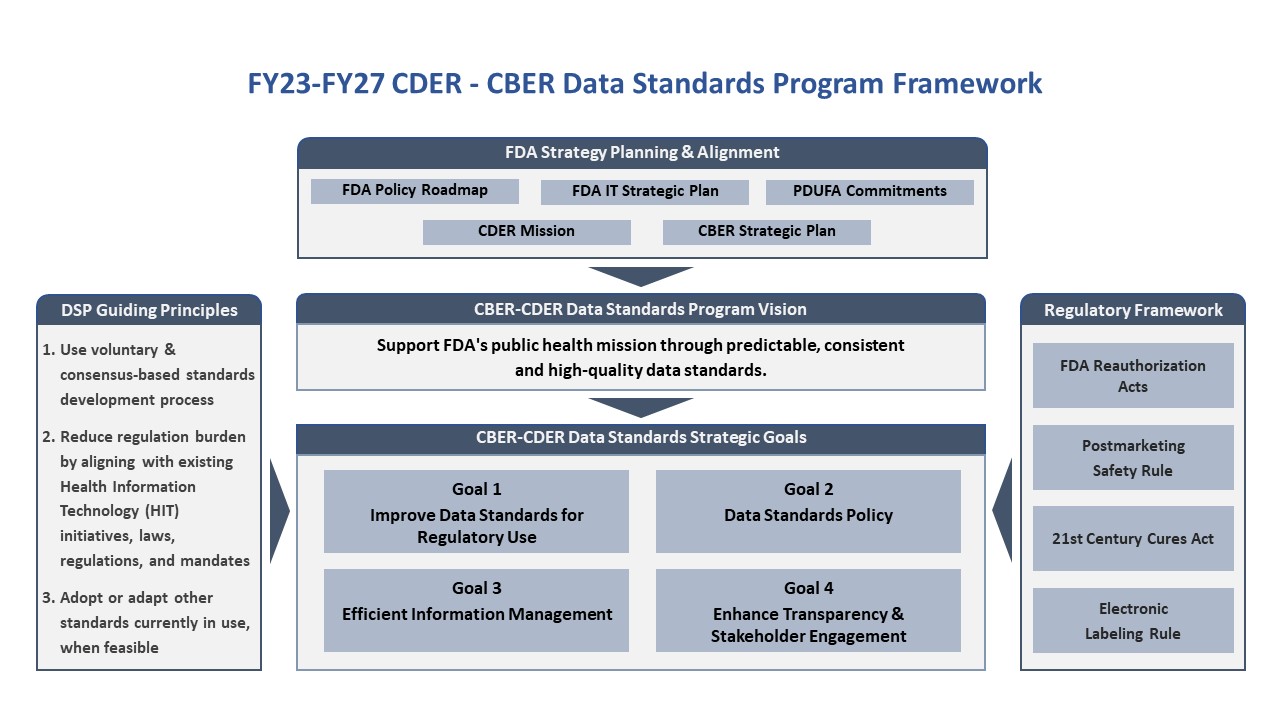
The Center for Drug Evaluation and Research (CDER) established the Data Standards Program in 2010. The program leads CDER’s efforts to standardize data and help FDA meet its performance goals under the Prescription Drug User Fee Act (PDUFA) commitment.
Under the PDUFA commitment, new data standards and electronic submission requirements have been established for submissions to CDER and the Center for Biologics Evaluation and Research (CBER).
Each year, CDER receives more than 300,000 submissions, amounting to millions of pieces of data. CDER reviews the data to bring lifesaving new drugs to market while protecting public health. The data can arrive in a wide variety of formats and even on paper, complicating the review process. To help simplify the process, more than a decade ago, FDA began requiring that certain submissions meet data standards.
The CDER Data Standards Program does the following:
- Identifies data standards needs
- Determines priorities for these needs
- Works with organizations that develop standards, industry, and other stakeholders to find or develop the necessary standards
Where current standard specifications are unavailable or inadequate, CDER plans to engage appropriate Standards Development Organizations (SDOs), as well as industry and other stakeholders, to develop new standards through SDO processes.
Learn more about the Data Standards Program Strategic Plan and Board.
Here are some of the accomplishments to date:
- Data Standards Program Strategic Plan and Board
- Published Final Guidance for Industry: Providing Regulatory Submissions in Electronic Format – eCTD Specifications
- Pharmaceutical Quality Chemistry Manufacturing and Control (PQ/CMC): Identify and standardize data elements, terminologies, and data structures to enable automation of key analyses of eCTD module 3 data. PQCMC
- ISO Identification of Medicinal Product (IDMP) standards: Adoption of IDMP standards to define medicinal product information for regional and global data sharing. IDMP
- SPL FHIR: Examining HL7 FHIR as an alternative to Structured Product Labeling (SPL) and determine if an HL7 FHIR implementation can support the same functionality and use cases as the current SPL standard.
- Conduct ongoing evaluations of where data quality can be further improved through the use of data standards, which is essential to develop large-scale analytics capabilities, automation, and other enhancements that will increase reviewer efficiency. SDSRW
A data standard is a set of rules on how a particular type of data should be structured, defined, formatted, or exchanged between computer systems. There are standards for everything, from how blood pressure is collected to how regulatory materials are submitted electronically to FDA.
Only some standards are required. See the FDA Data Standards Catalog (located on the Study Data Standards Resources page) for a complete list of standards currently supported or required by FDA. The catalog also indicates when future requirements will begin.
Data standards make submissions predictable, consistent, and in a form that an information technology system or a scientific tool can use.
- When submissions arrive in eCTD format, reviewers can easily find and access the information they need to review, whether it was part of the original submission or added later by the product sponsor.
- With data standards, reviewers can focus more on the scientific review rather than spending precious time navigating huge amounts of less-structured data.
- With study data standards, everyone is speaking the same language.
- Data standards open up new avenues for research.
- The Electronic Common Technical Document (eCTD) is the standard method for submitting applications, amendments, supplements, and reports to CDER and CBER.
- Study data standards describe a standard way to exchange clinical and nonclinical research data between computer systems.
- Product labeling submissions standardize the information included on labels of products regulated by FDA.
- Health information technology standards make it possible to use information from different electronic health records systems for research.
- Postmarketing safety reporting standards provide a standard way to submit adverse events and periodic reports to FDA.
See the FDA Data Standards Catalog (located on the Study Data Standards Resources page) for a complete list of standards currently supported or required by FDA. The catalog also indicates when future requirements will begin.
Questions or comments should be forwarded to the CDER Data Standards team at
CDERDataStandards@fda.hhs.gov.
The program welcomes input from stakeholders. Here are some ways you can get involved:
- Commenting on proposed data standards and draft guidances posted in the Federal Register
- Attending FDA’s meetings and webinars on data standards
- Collaborating with the Critical Path Institute, which works to improve data sharing and the drug development process