Guideline for Drug Master Files (DMF)
Center for Drug Evaluation and Research
Food and Drug Administration
Department of Health and Human Services
September 1989
For further information regarding the guideline please contact:
Food and Drug Administration
Center for Drug Evaluation and Research
Office of Drug Evaluation I
10903 New Hampshire Ave
White Oak Campus, Bldg 22
Silver Spring, MD 20993
TABLE OF CONTENTS
I. INTRODUCTION
II. DEFINITIONS
III. TYPES OF DRUG MASTER FILES
IV. SUBMISSIONS TO DRUG MASTER FILES
A. Transmittal Letters
- Original Submissions
- Amendments
B. Administrative Information
- Original Submissions
- Amendments
C. Drug Master File Contents
1. Types of Drug Master Files
a. Type I: Manufacturing Site, Facilities, Operating Procedures, and Personnel
b. Type II: Drug Substance, Drug Substance Intermediate, and Material Used in Their Preparation, or Drug Product
c. Type III: Packaging Material
d. Type IV: Excipient, Colorant, Flavor, Essence, or Material Used in Their Preparation
e. Type V: FDA Accepted Reference Information
2. General Information and Suggestions
A. Environmental Assessment
B. Stability
C. Format, Assembly, and Delivery
V. AUTHORIZATION TO REFER TO A DRUG MASTER FILE
A. Letter of Authorization to FDA
B. Copy to Applicant, Sponsor, or Other Holder
VI. PROCESSING AND REVIEWING POLICIES
A. Policies Related to Processing Drug Master Files
B. Drug Master File Review
VII. HOLDER OBLIGATIONS
A. Notice Required for Changes to a Drug Master File
B. Listing of Persons Authorized To Refer to a Drug Master File
C. Annual Update
D. Appointment of an Agent
E. Transfer of Ownership
IX. CLOSURE OF A DRUG MASTER FILE
GUIDELINE FOR DRUG MASTER FILES
I. INTRODUCTION
A Drug Master File (DMF) is a submission to the Food and Drug Administration (FDA) that may be used to provide confidential detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of one or more human drugs. The submission of a DMF is not required by law or FDA regulation. A DMF is submitted solely at the discretion of the holder. The information contained in the DMF may be used to support an Investigational New Drug Application (IND), a New Drug Application (NDA), an Abbreviated New Drug Application (ANDA), another DMF, an Export Application, or amendments and supplements to any of these.
A DMF is NOT a substitute for an IND, NDA, ANDA, or Export Application. It is not approved or disapproved. Technical contents of a DMF are reviewed only in connection with the review of an IND, NDA, ANDA, or an Export Application.
This guideline does not impose mandatory requirements (21 CFR 10.90(b)). It does, however, offer guidance on acceptable approaches to meeting regulatory requirements. Different approaches may be followed, but the applicant is encouraged to discuss significant variations in advance with FDA reviewers to preclude spending time and effort in preparing a submission that FDA may later determine to be unacceptable.
Drug Master Files are provided for in 21 CFR 314.420. This guideline is intended to provide DMF holders with procedures acceptable to the agency for preparing and submitting a DMF. The guideline discusses types of DMF's, the information needed in each type, the format of submissions to a DMF, the administrative procedures governing review of DMF's, and the obligations of the DMF holder.
DMF's are generally created to allow a party other than the holder of the DMF to reference material without disclosing to that party the contents of the file. When an applicant references its own material, the applicant should reference the information contained in its own IND, NDA, or ANDA directly rather than establishing a new DMF.
II. DEFINITIONS
For the purposes of this guideline, the following definitions apply:
II.1. Agency means the Food and Drug Administration.
II.2 Agent or representative means any person who is appointed by a DMF holder to serve as the contact for the holder.
II.3. Applicant means any person who submits an application or abbreviated application or an amendment or supplement to them to obtain FDA approval of a new drug or an antibiotic drug and any other person who owns an approved application (21 CFR 314.3 (b)).
II.4. Drug product means a finished dosage form, for example, tablet, capsule, or solution, that contains a drug substance, generally, but not necessarily, in association with one or more other ingredients (21 CFR 314.3 (b)).
II.5. Drug substance means an active ingredient that is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the human body, but does not include intermediates used in the synthesis of such ingredient (21 CFR 314.3 (b)).
II.6. Export application means an application submitted under section 802 of the Federal Food, Drug, and Cosmetic Act to export a drug that is not approved for marketing in the United States.
II.7. Holder means a person who owns a DMF.
II.8. Letter of authorization means a written statement by the holder or designated agent or representative permitting FDA to refer to information in the DMF in support of another person's submission.
II.9. Person includes individual, partnership, corporation, and association. (Section 201(e) of the Federal Food, Drug, and Cosmetic Act.)
II.10. Sponsor means a person who takes responsibility for and initiates a clinical investigation. The sponsor may be an individual or pharmaceutical company, governmental agency, academic institution, private organization, or other organization (21 CFR 312.3 (b)).
III. TYPES OF DRUG MASTER FILES
There are five types of DMF's:
Type I Manufacturing Site, Facilities, Operating Procedures, and Personnel
Type II Drug Substance, Drug Substance Intermediate, and Material Used in Their Preparation, or Drug Product
Type III Packaging Material
Type IV Excipient, Colorant, Flavor, Essence, or Material Used in Their Preparation
Type V FDA Accepted Reference Information
Each DMF should contain only one type of information and all supporting data. See Section IV.C of the guideline for more detailed descriptions of the kind of information desired in each type. Supporting information and data in a DMF can be cross referenced to any other DMF (see Part V).
IV. SUBMISSIONS TO DRUG MASTER FILES
Each DMF submission should contain a transmittal letter, administrative information about the submission, and the specific information to be included in the DMF as described in this section.
The DMF must be in the English language. Whenever a submission contains information in another language, an accurate certified English translation must also be included.
Each page of each copy of the DMF should be dated and consecutively numbered. An updated table of contents should be included with each submission.
IV. A. Transmittal Letters
The following should be included:
IV. A.1. Original Submissions
a. Identification of submission: Original, the type of DMF as classified in Section III, and its subject.
b. Identification of the applications, if known, that the DMF is intended to support, including the name and address of each sponsor, applicant, or holder, and all relevant document numbers.
c. Signature of the holder or the authorized representative.
d. Typewritten name and title of the signer.
IV. A. 2. Amendments
a. Identification of submission: Amendment, the DMF number, type of DMF, and the subject of the amendment.
b. A description of the purpose of submission, e.g., update, revised formula, or revised process.
c. Signature of the holder or the authorized representative.
d. Typewritten name and title of the signer.
IV. B. Administrative Information
Administrative information should include the following:
IV. B.1. Original Submissions
a. Names and addresses of the following:
(1) DMF holder.
(2) Corporate headquarters.
(3) Manufacturing/processing facility.
(4) Contact for FDA correspondence.
(5) Agent(s), if any.
b. The specific responsibilities of each person listed in any of the categories in Section a.
c. Statement of commitment.
A signed statement by the holder certifying that the DMF is current and that the DMF holder will comply with the statements made in it.
IV. B2. Amendments
a. Name of DMF holder.
b. DMF number.
c. Name and address for correspondence.
d. Affected section and/or page numbers of the DMF.
e. The name and address of each person whose IND, NDA, ANDA, DMF, or Export Application relies on the subject of the amendment for support.
f. The number of each IND, NDA, ANDA, DMF, and Export Application that relies on the subject of the amendment for support, if known.
g. Particular items within the IND, NDA, ANDA, DMF, and Export Application that are affected, if known.
IV. C. Drug Master File Contents
IV. C.1. Types of Drug Master Files
IV. C.1.a. Type I: Manufacturing Site, Facilities, Operating Procedures, and Personnel
A Type I DMF is recommended for a person outside of the United States to assist FDA in conducting on site inspections of their manufacturing facilities. The DMF should describe the manufacturing site, equipment capabilities, and operational layout.
A Type I DMF is normally not needed to describe domestic facilities, except in special cases, such as when a person is not registered and not routinely inspected.
The description of the site should include acreage, actual site address, and a map showing its location with respect to the nearest city. An aerial photograph and a diagram of the site may be helpful.
A diagram of major production and processing areas is helpful for understanding the operational layout. Major equipment should be described in terms of capabilities, application, and location. Make and model would not normally be needed unless the equipment is new or unique.
A diagram of major corporate organizational elements, with key manufacturing, quality control, and quality assurance positions highlighted, at both the manufacturing site and corporate headquarters, is also helpful.
IV. C.1.b.Type II: Drug Substance, Drug Substance Intermediate, and Material Used in Their Preparation, or Drug Product
A Type II DMF should, in general, be limited to a single drug intermediate, drug substance, drug product, or type of material used in their preparation.
IV. C.1.b.(1) Drug Substance Intermediates, Drug Substances, and Material Used in Their Preparation
Summarize all significant steps in the manufacturing and controls of the drug intermediate or substance. Detailed guidance on what should be included in a Type II DMF for drug substances and intermediates may be found in the following guidelines:
Guideline for Submitting Supporting Documentation in Drug Applications for the Manufacture of Drug Substances.
Guideline for the Format and Content of the Chemistry, Manufacturing, and Controls Section of an Application.
IV. C.1.b.(2) Drug Product
Manufacturing procedures and controls for finished dosage forms should ordinarily be submitted in an IND, NDA, ANDA, or Export Application. If this information cannot be submitted in an IND, NDA, ANDA, or Export Application, it should be submitted in a DMF. When a Type II DMF is submitted for a drug product, the applicant/sponsor should follow the guidance provided in the following guidelines:
Guideline for the Format and Content of the Chemistry, Manufacturing, and Controls Section of an Application.
Guideline for Submitting Documentation for the Manufacture of and Controls for Drug Products
Guideline for Submitting Samples and Analytical Data for Methods Validation
IV. C.1.c.Type III: Packaging Material
Each packaging material should be identified by the intended use, components, composition, and controls for its release. The names of the suppliers or fabricators of the components used in preparing the packaging material and the acceptance specifications should also be given. Data supporting the acceptability of the packaging material for its intended use should also be submitted as outlined in the "Guideline for Submitting Documentation for Packaging for Human Drugs and Biologics."
Toxicological data on these materials would be included under this type of DMF, if not otherwise available by cross reference to another document.
IV. C.1.d.Type IV Excipient, Colorant, Flavor, Essence, or Material Used in Their Preparation
Each additive should be identified and characterized by its method of manufacture, release specifications, and testing methods.
Toxicological data on these materials would be included under this type of DMF, if not otherwise available by cross reference to another document.
Usually, the official compendia and FDA regulations for color additives (21 CFR Parts 70 through 82), direct food additives (21 CFR Parts 170 through 173), indirect food additives (21 CFR Parts 174 through 178), and food substances (21 CFR Parts 181 through 186) may be used as sources for release tests, specifications, and safety. Guidelines suggested for a Type II DMF may be helpful for preparing a Type IV DMF. The DMF should include any other supporting information and data that are not available by cross reference to another document.
IV. C.1.e.Type V: FDA Accepted Reference Information
FDA discourages the use of Type V DMF's for miscellaneous information, duplicate information, or information that should be included in one of the other types of DMF's. If any holder wishes to submit information and supporting data in a DMF that is not covered by Types I through IV, a holder must first submit a letter of intent to the Drug Master File Staff (for address, see D.5.a. of this section). FDA will then contact the holder to discuss the proposed submission.
IV. C.2. General Information and Suggestions
IV. C.2.a. Environmental Assessment
Type II, Type III, and Type IV DMF's should contain a commitment by the firm that its facilities will be operated in compliance with applicable environmental laws. If a completed environmental assessment is needed, see 21 CFR Part 25.
IV. C.2.b. Stability
Stability study design, data, interpretation, and other information should be submitted, when applicable, as outlined in the "Guideline for Submitting Documentation for the Stability of Human Drugs and Biologics."
IV. D. Format, Assembly, and Delivery
IV. D.1.
An original and duplicate are to be submitted for all DMF submissions.
Drug Master File holders and their agents/representatives should retain a complete reference copy that is identical to, and maintained in the same chronological order as, their submissions to FDA.
IV. D.2.
The original and duplicate copies must be collated, fully assembled, and individually jacketed.
Each volume of a DMF should, in general, be no more than 2 inches thick. For multivolume submissions, number each volume. For example, for a 3 volume submission, the volumes would be numbered 1 of 3, 2 of 3, and 3 of 3.
IV. D.3.
U.S. standard paper size (8-1/2 by 11 inches) is preferred.
Paper length should not be less than 10 inches nor more than 12 inches. However, it may occasionally be necessary to use individual pages larger than standard paper size to present a floor plan, synthesis diagram, batch formula, or manufacturing instructions. Those pages should be folded and mounted to allow the page to be opened for review without disassembling the jacket and refolded without damage when the volume is shelved.
IV.D.4.
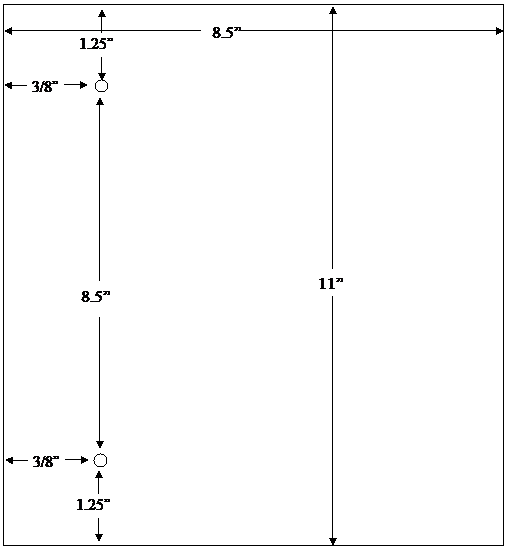
The agency's system for filing DMF's provides for assembly on the left side of the page. The left margin should be at least three fourths of an inch to assure that text is not obscured in the fastened area. The right margin should be at least one half of an inch. The submitter should punch holes 8 1/2 inches apart in each page. See the page measurements shown in the following figure:
IV.D.5. Delivery to FDA
IV.D.5.a.
Drug Master File submissions and correspondence should be addressed as follows:
Drug Master File Staff
Food and Drug Administration
5901-B Ammendale Rd.
Beltsville, MD 20705-1266
IV.D.5.b. Delivery charges to the above address must be prepaid.
V. AUTHORIZATION TO REFER TO A DRUG MASTER FILE
V. A. Letter of Authorization to FDA
Before FDA can review DMF information in support of an application, the DMF holder must submit in duplicate to the DMF a letter of authorization permitting FDA to reference the DMF. If the holder cross references its own DMF, the holder should supply in a letter of authorization the information designated by items 3, 5, 6, 7, and 8 of this section. The holder does not need to send a transmittal letter with its letter of authorization.
The letter of authorization should include the following:
- The date.
- Name of DMF holder.
- DMF number.
- Name of person(s) authorized to incorporate information in the DMF by reference.
- Specific product(s) covered by the DMF.
- Submission date(s) of 5, above.
- Section numbers and/or page numbers to be referenced.
- Statement of commitment that the DMF is current and that the DMF holder will comply with the statements made in it.
- Signature of authorizing official.
- Typed name and title of official authorizing reference to the DMF.
V. B. Copy to Applicant, Sponsor, or Other Holder
The holder should also send a copy of the letter of authorization to the affected applicant, sponsor, or other holder who is authorized to incorporate by reference the specific information contained in the DMF. The applicant, sponsor, or other holder referencing a DMF is required to include a copy of the DMF holder's letter of authorization in the application.
VI. PROCESSING AND REVIEWING POLICIES
VI. A. Policies Related to Processing Drug Master Files
VI. A.1.
Public availability of the information and data in a DMF is determined under 21 CFR Part 20, 21 CFR 314.420(e), and 21 CFR 314.430.
VI. A.2.
An original DMF submission will be examined on receipt to determine whether it meets minimum requirements for format and content. If the submission is administratively acceptable, FDA will acknowledge its receipt and assign it a DMF number.
If the submission is administratively incomplete or inadequate, it will be returned to the submitter with a letter of explanation from the Drug Master File Staff, and it will not be assigned a DMF number.
VI. B. Drug Master File Review
A DMF IS NEVER APPROVED OR DISAPPROVED.
The agency will review information in a DMF only when an IND sponsor, an applicant for an NDA, ANDA, or Export Application, or another DMF holder incorporates material in the DMF by reference. As noted, the incorporation by reference must be accompanied by a copy of the DMF holder's letter of authorization.
If FDA reviewers find deficiencies in the information provided in a DMF, a letter describing the deficiencies is sent to the DMF holder. At the same time, FDA will notify the person who relies on the information in the deficient DMF that additional information is needed in the supporting DMF. The general subject of the deficiency is identified, but details of the deficiency are disclosed only to the DMF holder. When the holder submits the requested information to the DMF in response to the agency's deficiency letter, the holder should also send a copy of the accompanying transmittal letter to the affected persons relying on the DMF and to the FDA reviewing division that identified the deficiencies. The transmittal letter will provide notice that the deficiencies have been addressed.
VII. HOLDER OBLIGATIONS
Any change or addition, including a change in authorization related to specific customers, should be submitted in duplicate and adequately cross referenced to previous submission(s). The reference should include the date(s), volume(s), section(s), and/or page number(s) affected.
VII. A. Notice Required for Changes to a Drug Master File
A holder must notify each affected applicant or sponsor who has referenced its DMF of any pertinent change in the DMF (21 CFR 314. 420(c)). Notice should be provided well before making the change in order to permit the sponsor/applicant to supplement or amend any affected application(s) as needed.
VII. B. Listing of Persons Authorized To Refer to a Drug Master File
VII. B.1.
A DMF is required to contain a complete list of persons authorized to incorporate information in the DMF by reference [21 CFR 314.420(d)]. The holder should update the list in the annual update. The updated list should contain the holder's name, DMF number, and the date of the update. The update should identify by name (or code) the information that each person is authorized to incorporate and give the location of that information by date, volume, and page number.
VII. B.2.
Any person whose authorization has been withdrawn during the previous year should be identified under a suitable caption.
VII. B.3.
If the list is unchanged on the anniversary date, the DMF holder should also submit a statement that the list is current.
VII. C. Annual Update
The holder should provide an annual report on the anniversary date of the original submission. This report should contain the required list as described in B.1., and should also identify all changes and additional information incorporated into the DMF since the previous annual report on the subject matter of the DMF. If the subject matter of the DMF is unchanged, the DMF holder should provide a statement that the subject matter of the DMF is current.
Failure to update or to assure FDA annually that previously submitted material and lists in the DMF remain current can cause delays in FDA review of a pending IND, NDA, ANDA, Export Application, or any amendment or supplement to such application; and FDA can initiate procedures for closure of the DMF (see Section IX).
VII. D. Appointment of an Agent
When an agent is appointed, the holder should submit a signed letter of appointment to the DMF giving the agent's name, address, and scope of responsibility (administrative and/or scientific). Domestic DMF holders do not need to appoint an agent or representative, although foreign DMF holders are encouraged to engage a U.S. agent.
VII. E. Transfer of Ownership
To transfer ownership of a DMF to another party, the holder should so notify FDA and authorized persons in writing. The letter should include the following:
- Name of transferee
- Address of transferee
- Name of responsible official of transferee
- Effective date of transfer
- Signature of the transferring official
- Typewritten name and title of the transferring official.
The new holder should submit a letter of acceptance of the transfer and an update of the information contained in the DMF, where appropriate. Any change relating to the new ownership (e.g., plant location and methods) should be included.
VIII. MAJOR REORGANIZATIONOF A DRUG MASTER FILE
A holder who plans a major reorganization of a DMF is encouraged to submit a detailed plan of the proposed changes and request its review by the Drug Master File Staff. The staff should be given sufficient time to comment and provide suggestions before a major reorganization is undertaken.
IX. CLOSURE OF A DRUG MASTER FILE
A holder who wishes to close a DMF should submit a request to the Drug Master File Staff stating the reason for the closure. See Section IV.D.5.a for the address.
The request should include a statement that the holder's obligations as detailed in Section VII have been fulfilled.
The Agency may close a DMF that does not contain an annual update of persons authorized to incorporate information in the DMF by reference and a list of changes made since the previous annual report. The holder will be notified of FDA's intent to close the DMF.
Many of the guidelines referred to in the text and a current list of available guidelines may be obtained from the following:
Legislative, Professional, and Consumer Affairs Branch (HFD-365)
Center for Drug Evaluation and Research
Food and Drug Administration
5600 Fishers Lane
Rockville, MD 20857
Copies of the Code of Federal Regulations (CFR) may be purchased from the following:
Superintendent of Documents
U.S. Government Printing Office
Washington, D.C. 20402