Advisory and Enforcement Actions Against Industry for Selling Tobacco Products to Underage Purchasers
On this page:
- Compliance Check Inspections
- Warning Letters
- Civil Money Penalty (CMP) Complaints
- No-Tobacco-Sale Order (NTSO) Complaints
To protect the health of future generations, FDA closely monitors industry compliance with tobacco laws and regulations under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and takes action when violations occur. FDA regulates tobacco products containing nicotine from any source, including non-tobacco nicotine (NTN), such as synthetic nicotine.
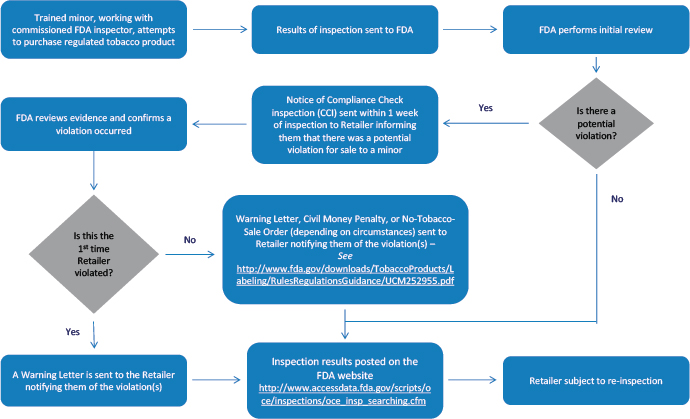
Compliance Check Inspections
FDA conducts inspections of tobacco product retailers to determine a retailer's compliance with federal laws and regulations, including the Federal Food, Drug, and Cosmetic Act (FD&C Act), as amended by the Tobacco Control Act, and our rules and regulations.
During Undercover Buy Inspections, the retailer is unaware an inspection is taking place. The inspector and underage purchaser will not identify themselves. Additional compliance education and information for retailers and small businesses are available on FDA’s Tobacco Compliance Webinars page.
Did You Know?
It is illegal for a retailer to sell any tobacco product – including cigarettes, cigars, and e-cigarettes – to anyone under 21.
How Do I Find Results from Compliance Check Inspections?
Results from compliance check inspections of tobacco retailers are available in CTP’s Tobacco Compliance Check Outcomes database.
The database lists which inspected retailers received a warning letter, a civil money penalty, a no-tobacco-sale order, or were found to have no observable violations. You can search by tobacco retailer name, city, state, zip code, or decision date.
Note: Absence of a retail establishment from this database does not imply compliance with all applicable statutory and regulatory requirements.
Warning Letters
FDA issues warning letters to traditional “brick and mortar” retail stores nationwide as well as online retailers and manufacturers the first time a tobacco compliance check inspection reveals a violation of the federal tobacco laws and regulations that FDA enforces. Failure to promptly and adequately correct all violations and ensure compliance with all applicable laws and regulations may lead to enforcement actions, including civil money penalty or no-tobacco-sale order complaints. All warning letters issued as the result of compliance check inspections of tobacco retailers prior to October 1, 2016, have been archived via the FDA Web Archive.
If you have any questions about warning letters, contact the Center for Tobacco Products (CTP) at 1-877-CTP-1373 or via email to the address listed in your warning letter: CTP-Compliance-WL-Correspondence@fda.hhs.gov or CTPCompliance@fda.hhs.gov.
How Do I Search for Warning Letters?
On FDA’s warning letters page, you can find all of these warning letters by entering “Center for Tobacco Products” in the “Issuing Office” box in the “Filter by” section of the search tool.
Civil Money Penalty (CMP) Complaints
A civil money penalty (CMP) complaint is used to initiate an administrative legal action against a tobacco product retailer that can result in a monetary penalty, called a civil money penalty.
The Tobacco Control Act provides that the amount of a CMP for violations of the regulations in part 1140 shall not exceed certain amounts, depending on the number of previous violations, the time period in which the violations occurred, and other factors (see section 103(q)(2)(A) of the Tobacco Control Act). The current maximum amounts for retailer CMPs are:
| Number of Regulation Violations | CMP Amount |
|---|---|
| 1 | $0 (CTP will send a Warning Letter) |
| 2 within a 12-month period | $356 |
| 3 within a 24-month period | $709 |
| 4 within a 24-month period | $2,846 |
| 5 within a 36-month period | $7,115 |
| 6 within a 48-month period | $14,232 |
Additionally, the maximum penalty amount for violating a requirement of the FD&C Act relating to tobacco products is $21,348 for a single violation. FDA intends to seek the maximum allowed by law in CMP cases relating to unauthorized tobacco products.
Under the Federal Civil Penalties Inflation Adjustment Act Improvements Act of 2015 (Pub. L. No. 114-74), the Department of Health and Human Services (HHS) is required to adjust the amounts annually for inflation no later than January 15th each year. Read the rule, Adjustment of Civil Monetary Penalties for Inflation, for more information. See the Federal Register notice with the 2024 adjusted amounts.
FDA files CMP complaints against retailers and manufacturers when subsequent violations of the tobacco regulations and/or other requirements relating to tobacco products in the FD&C Act are observed during an inspection.
All tobacco retail CMP complaints filed by CTP are listed in the searchable Tobacco Compliance Check Outcomes database.
If you are a retail establishment or manufacturer that has received a CMP complaint and wish to settle the case, you can submit the Acknowledgement Form and pay your CMP online.
For more information about CMP complaints, view FDA guidance documents and webinars.
No-Tobacco-Sale Order (NTSO) Complaints
Under the law, the FDA may pursue a no-tobacco-sale order (NTSO) complaint against retailers that have a total of five or more repeated violations of certain restrictions within 36 months. Retailers are prohibited from selling regulated tobacco products at the specified location during the period of the NTSO.
Retailers who receive an NTSO complaint from the FDA may either:
- Enter into a settlement agreement with the agency that results in a final order issued by the Administrative Law Judge (ALJ), or
- Respond to the court with an Answer and choose to go to a full hearing before an ALJ.
In determining the period of an NTSO, FDA considers the nature, circumstances, extent, and gravity of the violations and, with respect to the violator, ability to pay, effect on ability to continue to do business, any history of prior such violations, the degree of culpability, and such other matters as justice may require.
When FDA conducts unannounced compliance check inspections during the period of the NTSO, for the limited purpose of confirming the retailer’s compliance with the NTSO complaint, the results are not included in the searchable inspection database.
View a list of all NTSOs imposed by an ALJ, along with the effective dates of each NTSO.
For more information about NTSOs, view FDA guidance documents and webinars.
FDA Age Calculator
Download the “FDA Age Calculator,” a voluntary smartphone application to help retailers comply with federal, state, and local age restrictions for selling tobacco products.