Drug Trials Snapshots: SPINRAZA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to SPINRAZA Prescribing Information for complete information.
SPINRAZA (nusinersen)
(spin-RA-za)
Biogen Inc.
Approval date: December 23, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
SPINRAZA is a drug for the treatment of spinal muscular atrophy (SMA).
Spinal muscular atrophy is a rare genetic disease that affects mostly children and young adults. It is caused by a low level of protein in the motor neuron that are responsible for normal muscle functioning. The lack of protein causes motor neuron loss, progressive muscle weakness and may lead to premature death from respiratory failure.
How is this drug used?
SPINRAZA is given by a health care professional by needle injection directly into the fluid surrounding the spinal cord. This is known as an intrathecal injection. The first three doses are given every 14 days, followed by a fourth dose 30 days later. Injections are then administered every 120 days.
What are the benefits of this drug?
Approval was based on a trial where forty percent of patients treated with SPINRAZA achieved improvement in motor milestones, whereas none of the control patients did.
More trials are ongoing to assess the clinical benefit.
What are the benefits of this drug (results of trials used to assess efficacy)?
The primary endpoint assessed at the time of interim analysis was the proportion of responders defined as patients with an improvement in motor milestones according to Section 2 of the Hammersmith Infant Neurologic Exam (HINE).
Although not statistically controlled for multiple comparisons at the interim analysis, the trial also assessed treatment effects on the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND), which is an evaluation of motor skills in patients with infantile-onset SMA.
The efficacy results are displayed in Table 2.
Table 2. Motor Milestone Response and CHOP-INTEND Results
| Endpoint | SPINRAZA-treated patients (n=52)1 | Sham-control patients (n=30)1 | |||
|---|---|---|---|---|---|
| Motor Milestone (HINE Section 2) | |||||
| Achievement of a motor milestone response | 21 (40%) p> |
0 (0%) | |||
| CHOP-INTEND Improvement from Baseline2 | |||||
| At least 4-points | 33 (63%) | 1 (3%) | |||
| CHOP-INTEND Worsening from Baseline2 | |||||
| At least 4-points | 2 (4%) | 12 (40%) | |||
1Analyses included all subjects who were alive with the opportunity for at least a 6-month (Day 183) assessment and all subjects who died or withdrew from the study at the time of the interim analysis
2Not statistically controlled for multiple comparisons at interim analysis
SPINRAZA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The differences in response to SPINRAZA between males and females could not be determined due to the overall small number of patients in the trial.
- Race: The number of patients in the overall clinical trial was small, and the majority of patients were white; therefore, differences in response to SPINRAZA among races could not be determined.
- Age: The differences among age groups could not be determined due to overall small number of patients. In addition, SMA is mostly a disease of children and young adults; therefore, it is not known how well SPINRAZA works in elderly patients.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The differences in response to SPINRAZA among sex, race, and age groups could not be determined due to the overall small number of patients.
What are the possible side effects?
SPINRAZA may cause serious side effects including low blood platelet count and toxicity to the kidneys.
The most common side effects are respiratory infections and constipation.
What are the possible side effects (results of trials used to assess safety)?
In the controlled study, 6 of 56 (11%) SPINRAZA-treated patients with normal or above normal platelet levels at baseline developed a platelet level below the lower limit of normal, compared to 0 of 28 sham-procedure control patients. No patient had a platelet count less than 50,000 cells per microliter in this study and no patient developed a sustained low platelet count despite continued drug exposure.
In a clinical study (mean treatment exposure 7 months), 17 of 51 (33%) SPINRAZA-treated patients had elevated urine protein, compared to 5 of 25 (20%) sham-control patients. In a group of later-onset SMA patients (mean treatment exposure 34 months), 36 of 52 (69%) had elevated urine protein.
The table below summarizes the most common adverse reactions that occurred in patients from the controlled trial.
Table 3. Adverse Reactions that Occurred in at Least 5% of SPINRAZA Patients and Occurred at Least 5% More Frequently or At Least 2 Times as Frequently Than in Control Patients in the Controlled Trial in Infants with Symptomatic SMA
| Adverse Reactions | SPINRAZA 12 mg1 N=80 % |
Sham-Procedure Control N=41 % |
|---|---|---|
| Lower respiratory infection2 | 43 | 29 |
| Upper respiratory infection3 | 39 | 34 |
| Constipation | 30 | 22 |
| Teething | 14 | 7 |
| Upper respiratory tract congestion | 6 | 2 |
| Aspiration | 5 | 2 |
| Ear infection | 5 | 2 |
| Scoliosis | 5 | 2 |
1 Four loading doses followed by 12 mg once every 4 months
2 Includes pneumonia, bronchiolitis, pneumonia viral, respiratory syncytial virus bronchiolitis, lower respiratory tract infection, pneumonia bacterial, bronchitis, bronchitis viral, pneumonia moraxella, pneumonia parainfluenzae viral, lower respiratory tract infection viral, lung infection, pneumonia influenzal, pneumonia pseudomonal, pneumonia respiratory syncytial viral
3 Includes upper respiratory tract infection, nasopharyngitis, rhinitis, pharyngitis, or tracheitis
SPINRAZA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects and the most common types of side effects were similar between males and females.
- Race: The number of patients in the overall clinical trial was small, and the majority of patients were white; therefore, differences in side effects among races could not be determined.
- Age: The occurrence of side effects between different age groups could not be determined because of the overall small number of patients. In addition, SMA is mostly a disease of children and young adults; safety in elderly patients has not been evaluated.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The differences in side effects among race and age groups could not be determined because of the small number of patients at the time of an early trial assessment. There do not appear to be differences in occurrence of side effects between males and females in the controlled trial, although this observation is limited by the small sample size.
Table below summarizes incidence of adverse events (AEs) by subgroup in patients from the controlled trial. The subgroup analyses are exploratory and limited by the small sample size.
Table 4. Occurrence of Any Adverse Event by Subgroup
| Any AE | SPINRAZA N=80 m/n* (%) |
Sham-Procedure Control N=41 m/n* (%) |
|---|---|---|
| Sex | ||
| Male | 33/37 (89) | 15/17 (88) |
| Female | 39/43 (91) | 23/24 (96) |
| Age Group | ||
| 90> | 5/6 (83) | 2/2 (100) |
| ≥90 days | 67/74 (91) | 36/39 (92) |
| Race | ||
| White | 62/68 (91) | 33/36 (92) |
| Non-White | 10/12 (83) | 4/4** (100) |
*m/n= number of patients with adverse event (m) in the subgroup (n)
** one patient with non-reported race excluded
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
FDA approved SPINRAZA based primarily on one controlled clinical trial of 121 infants with SMA. The trial was conducted in the United States, Canada, France, Germany, Spain, and Japan. All 121 patients were considered in the assessment of SPINRAZA’s safety (safety population). Patients were enrolled in the trial for different lengths of time, 82 of the 121 patients were in the trial study long enough to be considered in the assessment of the drug’s benefits.
FDA also considered other trials where SPINRAZA was given to patients of various ages.
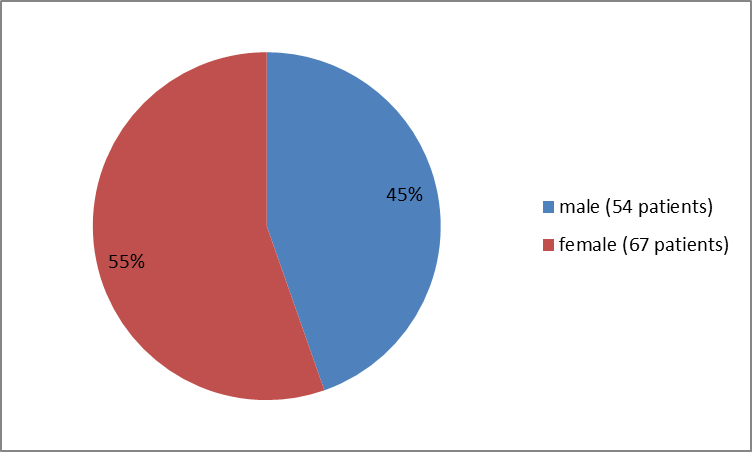
The figure below summarizes patients who participated in the controlled trial by sex.
Figure 1. Baseline Demographics by Sex (safety population)
Clinical trial data
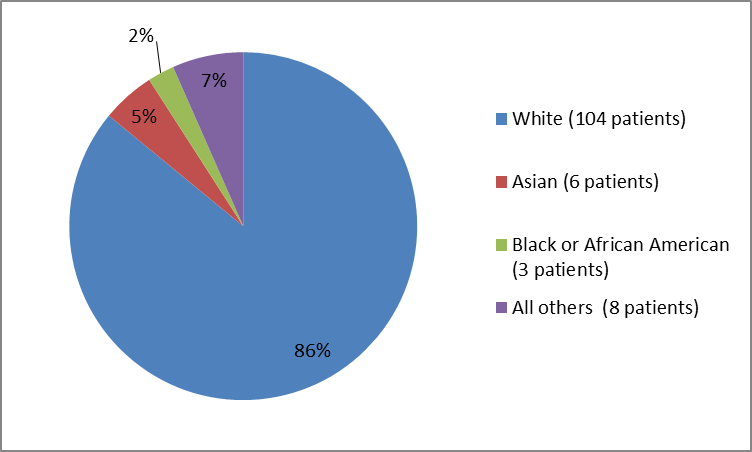
Figure 2 and Table 1 below summarize the percentage of patients in the controlled trial by race.
Figure 2. Baseline Demographics by Race (safety population)
Clinical trial data
Table 1. Baseline Demographics by Race (safety population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 104 | 86 |
| Asian | 6 | 5 |
| Black or African American | 3 | 2 |
| Multiple | 3 | 2 |
| Other | 4 | 3 |
| Not reported | 1 | less than 1 |
Clinical trial data
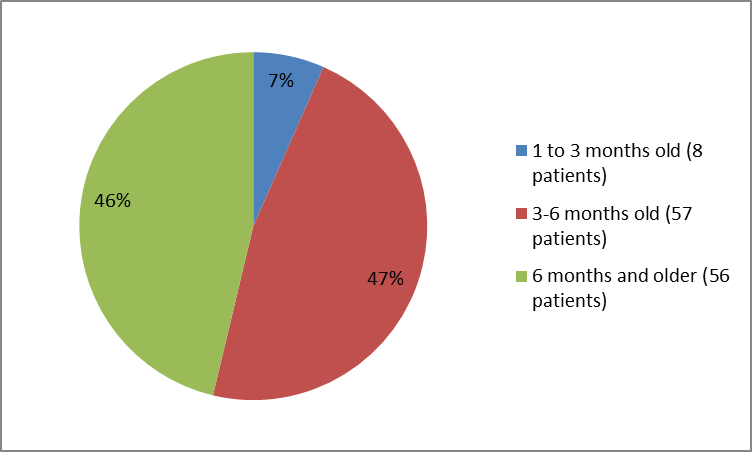
The figure below summarizes the percentage of patients in the controlled trial by age group.
Figure 3. Baseline Demographics by Age (safety population)
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in the controlled clinical trial. Presented is safety population defined as any patient who either received SPINRAZA or underwent the sham procedure.
Table 5. Baseline Demographics (safety population)
| Demographic Parameters | SPINRAZA N=80 n (%) |
Sham (N=41) n (%) |
Total N=121 n (%) |
|---|---|---|---|
| Sex | |||
| Male | 37 (46) | 17 (41) | 54 (45) |
| Female | 43 (54) | 24 (59) | 67 (55) |
| Age (days) | |||
| Mean | 163.4 | 180.5 | 169.2 |
| Median | 164.5 | 205 | 175 |
| Min, max | 52, 242 | 30, 262 | 30, 262 |
| Age Groups (days) | |||
| > | 0 | 0 | 0 |
| 30 to > | 6 (8) | 2 (5) | 8 (7) |
| 90-180 | 43 (54) | 14 (34) | 57 (47) |
| ≥180 | 31 (39) | 25 (61) | 56 (46) |
| Race | |||
| White | 68 (85) | 36 (88) | 104 (86) |
| Asian | 5 (6) | 1 (2) | 6 (5) |
| Black or African American | 3 (4) | 0 (0) | 3 (2) |
| Multiple | 1 (1) | 2 (5) | 3 (2) |
| Other | 3 (4) | 1 (2) | 4 (3) |
| Not reported | 0 (0) | 1 (2) | 1 (> |
| Ethnicity | |||
| Hispanic or Latino | 12 (15) | 4 (10) | 16 (13) |
| Not Hispanic or Latino | 68 (85) | 37 (90) | 105 (87) |
| Region | |||
| North America | 38 (48) | 22 (54) | 60 (50) |
| Europe | 30 (38) | 17 (41) | 47 (39) |
| Asia-Pacific | 12 (15) | 2 (5) | 14 (12) |
Clinical trial data
How were the trials designed?
There was one trial that evaluated the benefit and side effects of SPINRAZA.
Patients were randomly assigned to receive SPINRAZA into the fluid surrounding the spinal cord, or undergo a mock procedure without drug injection (a skin prick). Neither the parents nor the health care providers knew whether the treatment (or skin prick) was given until the trial ended.
The benefit was measured by the percentage of patients with improvement in motor milestones, such as head control, sitting, ability to kick in supine position, rolling, crawling, standing and walking.
The trial was stopped after an early analysis of benefit (called an interim analysis) was completed. After the analysis, the trial design was changed and all the patients were switched to receive SPINRAZA.
How were the trials designed?
The safety and efficacy of SPINRAZA were evaluated in a multicenter, randomized, double-blind, sham-procedure controlled trial in 121 symptomatic infants ≤ 7 months of age, diagnosed with SMA (symptom onset before 6 months of age). Patients were randomized 2:1 to receive either SPINRAZA or sham-control, with a length of treatment ranging from 6 to 442 days (median 162).
The primary clinical efficacy endpoint at the time of interim analysis was the proportion of patients achieving a level of improvement in motor milestones using the Hammersmith Infant Neurological Examination (HINE) Section 2: a measure of the achievement of 8 motor milestones. A treatment responder was defined as any patient with at least a 2-point increase (or maximal score of 4) in ability to kick (consistent with improvement by at least 2 milestones), or at least a 1-point increase in the motor milestones of head control, rolling, sitting, crawling, standing, or walking (consistent with improvement by at least 1 milestone) who also demonstrated improvement in more categories than worsening. (Change in voluntary grasp was not included in the endpoint).
Efficacy was assessed by comparing the motor milestone responders of SPINRAZA-treated patients with patients who received a sham procedure.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.