Drug Trials Snapshots: KYBELLA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the KYBELLA Prescribing Information for complete information.
KYBELLA (deoxycholic acid) injection
kye be’ lah
Kythera Biopharmaceuticals, Inc.
Approval date: April 29, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
KYBELLA is a treatment for adults who appear to have too much fat below the chin, commonly called “double chin.”
How is this drug used?
KYBELLA is given by a health provider as an injection into the fat below the chin.
What are the benefits of this drug?
KYBELLA destroys fat cells which reduces the appearance of fat below the chin.
title="MORE INFO" open="no"
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the co-primary efficacy assessments for each trial separately. These were based on at least 2-grade and at least 1-grade improvements in submental convexity or fullness on the composite of clinician-reported and patient-reported ratings of submental fat 12 weeks after final treatment.
Table 2. ≥ 2-Grade and ≥ 1-Grade Composite Clinician and Patient Response 12 Weeks After Final Treatment
|
Endpoint |
Trial 1 | Trial 2 | ||
|---|---|---|---|---|
| KYBELLA (N=256) |
Placebo (N=250) |
KYBELLA (N=258) |
Placebo (N=258) |
|
|
2-Grade Composite Responsea |
13.4% | <> | 18.6% | 3% |
|
1-Grade Composite Responseb |
70% | 18.6% | 66.5% | 22.2% |
aat least 2 grade reduction on both, the clinician–reported and patient-reported ratings of submental fat
bat least 1 grade reduction on both, the clinical–reported and patient-reported ratings of submental fat
Source: KYBELLA Prescribing Information, Section 14, Table 2
title="MORE INFO" open="no"
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The majority of patients in the trials were female. KYBELLA appeared to be similarly effective in men and women.
- Race: The majority of patients in the trials were white. Therefore, differences in response to KYBELLA among races could not be determined.
- Age: The majority of patients in the trials were 19 to 65 years old. Differences in response to KYBELLA among age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
The table below summarizes the response to KYBELLA for the combined populations from the two trials. The analysis is done with a multiple imputation method for missing data.
Table 3. Subgroup Analysis of Primary Endpoint for Two Trials (Intent to Treat Population)
Subgroup |
KYBELLA (N=514) |
Placebo (N=508) |
||
|---|---|---|---|---|
| n (%) | Total, n | n (%) | Total, n | |
| Overall Response/All patients ITT | 82 (16) | 514 | 8 (2) | 508 |
| Sex | ||||
| Male | 7 (9) | 80 | 0 | 76 |
| Female | 75 (17) | 434 | 8 (2) | 432 |
| Age Group | ||||
| <17> | 0 | 0 | 0 | 0 |
| >=17 - <65> | 78 (15) | 505 | 8 (2) | 503 |
| >=65 years | 4 (44) | 9 | 0 | 5 |
| >=75 years | 0 | 0 | 0 | 0 |
| Race | ||||
| White | 80 (18) | 440 | 5 (1) | 449 |
| Black or African American | 1 (2) | 48 | 2 (6) | 34 |
| Asian | 0 | 11 | 0 | 10 |
| American Indian or Alaska Native | 0 | 1 | 0 | 4 |
| Native Hawaiian or Other Pacific Islander | 0 | 3 | 0 | 2 |
| Multiple | 0 | 4 | 0 | 0 |
| Other | 1 (14) | 7 | 0 | 9 |
Source: Review Division
What are the possible side effects?
KYBELLA can cause serious but temporary side effects including nerve injury in the jaw and difficulty swallowing. Nerve injury can lead to an uneven smile or weakness of the face muscles. Difficulty swallowing occurs because of swelling and pain in the area below the chin.
The most common side effects of KYBELLA include swelling, bruising, pain, numbness, redness and hardness in the area under the chin or lower jaw that was treated.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions for the two pooled trials. The population represented is the Safety Population, which includes any patient who received at least one dose of study drug.
Table 4. Adverse Reactions in the Pooled Trials*
Adverse Reaction |
KYBELLA (N=513) n (%) |
Placebo (N=506) n (%) |
|---|---|---|
| Injection site reactions | 492 (96) | 411 (81) |
| edema/swelling | 448 (87) | 218 (43) |
| hematoma/bruising | 368 (72) | 353 (70) |
| pain | 356 (70) | 160 (32) |
| numbness | 341 (66) | 29 (6) |
| erythema | 136 (27) | 91 (18) |
| induration | 120 (23) | 13 (3) |
| paresthesia | 70 (14) | 20 (4) |
| nodule | 68 (13) | 14 (3) |
| pruritus | 64 (12) | 30 (6) |
| skin tightness | 24 (5) | 6 (1) |
| site warmth | 22 (4) | 8 (2) |
| nerve injurya | 20 (4) | 1 (<> |
| Headache | 41 (8) | 20 (4) |
| Orpharyngeal pain | 15 (3) | 7 (1) |
| Hypertension | 13 (3) | 7 (1) |
| Nausea | 12 (2) | 3 (1) |
| Dysphagia | 10 (2) | 1 (<> |
*adverse reactions that occurred in ≥2% of KYBELLA treated subjects and at greater incidence than placebo
amarginal mandibular nerve paresis
Source: KYBELLA Prescribing Information, Section 6, Table 1
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The majority of patients in the trials were female. The risk of overall side effects appeared to be similar in males and females.
- Race: The majority of patients in the trials were white. Differences in side effects among races could not be determined.
- Age: The majority of patients in the trials were 19 to 65 years old. Differences in side effects among age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The table below summarizes adverse events while on treatment by subgroup for the pooled trials.
Table 5. Subgroup Analysis of all Adverse Events While on Treatment (Safety Population)
| Subgroup | KYBELLA (N=513) |
Placebo (N=506) |
||
|---|---|---|---|---|
| n (%) | Total, n | n (%) | Total, n | |
| Any TEAEs* | 499 (97) | 513 | 454 (90) | 506 |
| Sex | ||||
| Male | 77 (96) | 80 | 60 (79) | 76 |
| Female | 422 (97) | 433 | 394 (92) | 430 |
| Age Group (years) | ||||
| <> | 0 | 0 | ||
| >=17 - <> | 490 (97) | 504 | 449 (90) | 501 |
| >=65 | 9 (100) | 9 | 5 (100) | 5 |
| >=75 | 0 | 0 | ||
| Race | ||||
| White | 433 (97) | 439 | 403 (90) | 447 |
| Black or African American | 42 (88) | 48 | 29 (85) | 34 |
| Asian | 10 (91) | 11 | 8 (80) | 10 |
| American Indian or Alaska Native | 1 (100) | 1 | 4 (100) | 4 |
| Native Hawaiian or Other Pacific Islander | 3 (100) | 3 | 2 (100) | 2 |
| Multiple | 3 (75) | 4 | 0 | 0 |
| Other | 7 (100) | 7 | 8 (89) | 9 |
*TEAE=treatment-emergent adverse event
Source: Review Division
The table below summarizes side effects related to the injection of drug in males and females for the pooled trials.
Table 6. Injection Site Reactions by Sex in the Pooled Trials
| Injection Site Reaction | KYBELLA (N=513) |
Placebo (N=506) |
||
|---|---|---|---|---|
| Female (N=433) n (%) |
Male (N=80) n (%) |
Female (N=430) n (%) |
Male (N=76) n (%) |
|
| swelling | 376 (87) | 72 (90) | 189 (44) | 29 (38) |
| bruising | 314 (73) | 54 (66) | 321 (75) | 32 (42) |
| pain | 305 (70) | 51 (64) | 143 (33) | 17 (22) |
| numbness | 281 (65) | 60 (75) | 27 (6) | 2 (3) |
| redness | 123 (28) | 13 (16) | 80 (18) | 11(14) |
| hardness | 108 (25) | 12 (15) | 11 (3) | 2 (3) |
Source: Review Division
WHO WAS IN THE CLINICAL TRIALS
Who participated in the clinical trials?
The FDA approved KYBELLA based on evidence from two clinical trials that enrolled a total of 1,022 adults with the appearance of moderate or severe amounts of fat located beneath the chin or lower jaw known as submental fat. The trials were conducted at 70 sites in the United States and Canada.
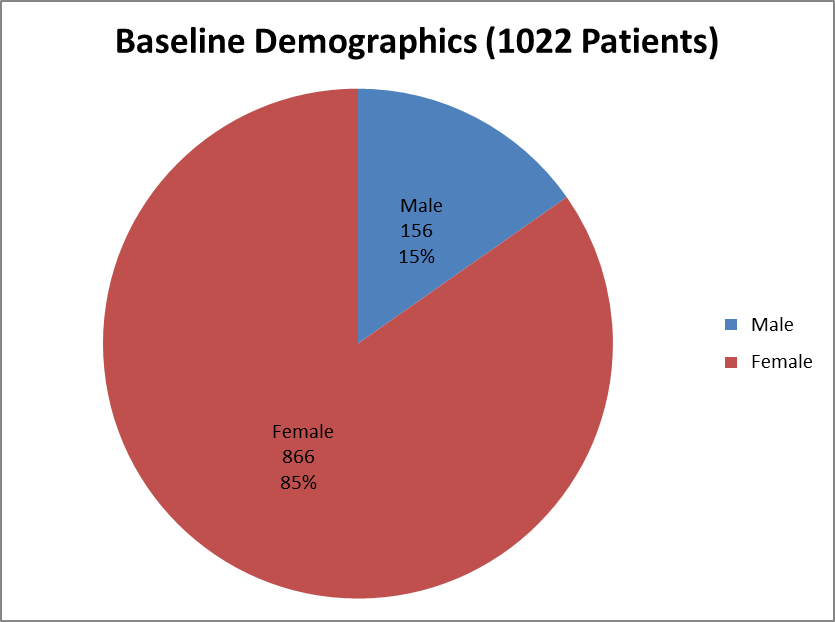
Figure 1 summarizes how many men and women in were enrolled in the clinical trials.
Figure 1. Baseline Demographics by Sex
Source: Review Division
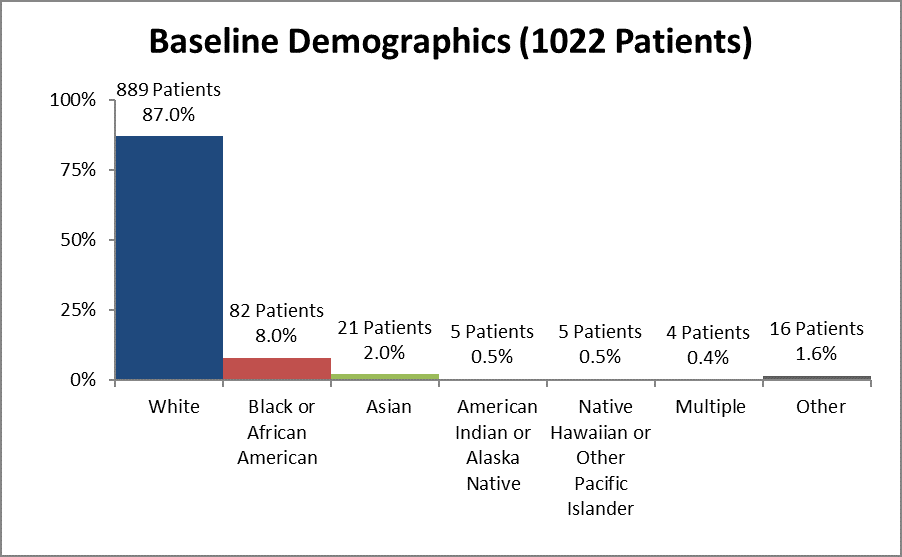
Figure 2 and Table 1 summarizes how many patients by race were enrolled in the clinical trials.
Figure 2. Baseline Demographics by Race
Source: Review Division
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 889 | 87% |
| Black or African American | 82 | 8% |
| Asian | 21 | 2% |
| American Indian or Alaska Native | 5 | 0.5% |
| Native Hawaiian or Other Pacific Islander | 5 | 0.5% |
| Multiple | 4 | 0.4% |
| Other | 16 | 1.6% |
Source: Review Division
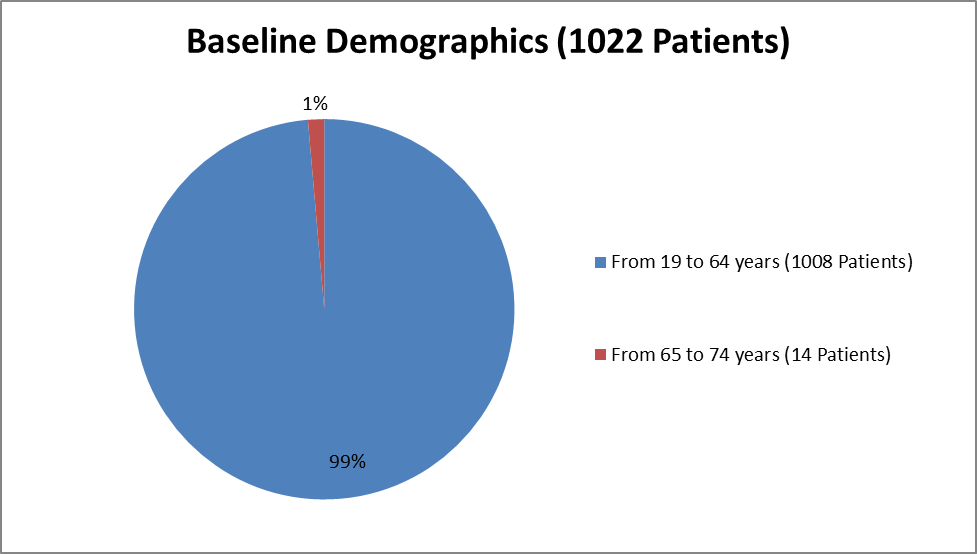
The figure below summarizes how many patients by age were enrolled in the clinical trials.
Figure 3. Baseline Demographics by Age
Source: Review Division
Who participated in the trials?
The table below summarizes baseline demographics for the pooled efficacy population for the two trials.
Table 7. Baseline Demographics for the Trials (Efficacy Population)
Demographic Parameters |
KYBELLA (N=514) n (%) |
Placebo (N=508) n (%) |
Total (N=1022) n (%) |
|---|---|---|---|
| Sex | |||
| Male | 80 (16) | 76 (15) | 156 (15) |
| Female | 434 (84) | 432 (85) | 866 (85) |
| Age | |||
| Mean years (SD) | 48.9 (9.3) | 48.5 (9.1) | 48.6 |
| Median (years) | 50 | 50 | 50 |
| Min, Max (years) | 19-65 | 21-65 | 19-65 |
| Age Group (years) | |||
| <> | 0 | 0 | 0 |
| >=17 - <> | 505 (98) | 503 (99) | 1008 (99) |
| >=65 | 9 (2) | 5 (1) | 14 (1) |
| >=75 | 0 | 0 | 0 |
| Race | |||
| White | 440 (86) | 449 (88) | 889 (87) |
| Black or African American | 48 (9) | 34 (7) | 82 (8) |
| Asian | 11(2) | 10 (2) | 21 (2) |
| American Indian or Alaska Native | 1 (<> | 4 (1) | 5 (<> |
| Native Hawaiian or Other Pacific Islander | 3 (<> | 2 (<> | 5 (<> |
| Multiple | 4 (1) | 0 | 4 (<> |
| Other | 7 (1) | 9 (2) | 16 (2) |
| Ethnicity | |||
| Hispanic or Latino | 68 (13) | 56 (11) | 124 (12) |
| Not Hispanic or Latino | 446 (87) | 452 (89) | 898 (88) |
| Region | |||
| United States | 451 (88%) | 444 (87%) | 895 (88%) |
| Canada | 63 (12%) | 64 (13%) | 127 (12%) |
Source: Review Division
How were the trials designed?
There were two trials that evaluated both the benefit and the side effects of KYBELLA. In each trial, patients were randomly assigned to receive KYBELLA or a placebo injection for a total of 6 treatments. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
The trials measured the change in the appearance of “double chin.”
How were the trials designed?
Two randomized, multi-center, double-blind, placebo-controlled trials of identical design were conducted to evaluate KYBELLA for use in improvement in the appearance of convexity or fullness associated with submental fat. The trials enrolled healthy adults with moderate or severe convexity or fullness associated with submental fat (i.e., grade 2 or 3 on 5-point grading scales, where 0=none and 4=extreme), as judged by both the clinician and the patient. Subjects received up to 6 treatments with KYBELLA or placebo at no less than 1 month intervals.
The co-primary efficacy assessments were based on at least 2-grade and at least 1-grade improvements in submental convexity or fullness on the composite of clinician-reported and patient-reported ratings of submental fat 12 weeks after final treatment.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION
MEDICAL REVIEW