Drug Trials Snapshot: TAGRISSO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the TAGRISSO Prescribing Information for complete information.
TAGRISSO (osimertinib)
Tuh-GRISS-oh
AstraZeneca
Approval date: November 13, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TAGRISSO is a drug used to treat patients with a type of lung cancer called advanced non-small cell lung cancer (NSCLC). It is to be used only in patients whose cancer has a specific mutation of the epidermal growth factor receptor, called T790M, and whose disease has gotten worse after treatment with another specific treatment called EGFR-inhibitor therapy.
How is this drug used?
TAGRISSO is a tablet taken by mouth once a day.
What are the benefits of this drug?
TAGRISSO had a significant effect on reducing tumor size on over half of patients who were treated.
What are the benefits of this drug (results of trials used to assess efficacy)?
Efficacy results are summarized below for the two clinical trials, summarized as Trial 1 and Trial 2.
Table 2. Efficacy Results for BICR for the Two Clinical Trials
Efficacy Parameter |
Trial 1 (N=201) |
Trial 2 (N=210) |
Overall2 (N=411) |
|---|---|---|---|
| Objective Response Rate1 (95% CI) |
57% (50, 64) |
61% (54, 68) |
59% (54, 64) |
| Complete Response | 0 | 1% | 0.5% |
| Partial Response | 57% | 60% | 59% |
1Objective response rate determined by RECIST v1.1 as assessed by BICR
2Pooled analysis of Trials 1 and 2.
TAGRISSO Prescribing Information, Table 4
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex:TAGRISSO was similarly effective in men and women.
- Race: TAGRISSO was similarly effective in all races studied.
- Age:TAGRISSO was similarly effective in patients below and above 65 years old.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
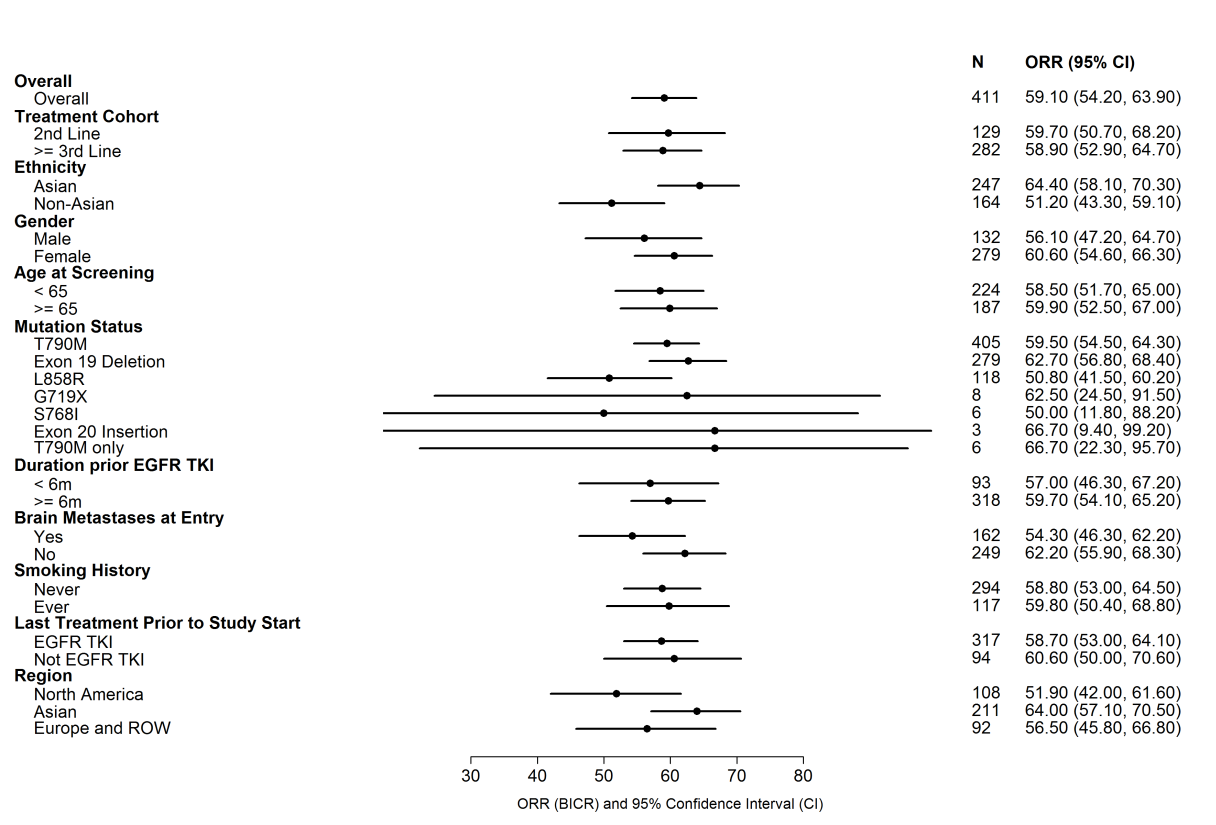
Subgroup analysis for efficacy for the pooled pivotal trials is summarized in the Forest plot below.
Figure 4. Subgroup Analysis per BICR assessment for Pooled Pivotal Trials (AURA Extension and AURA2, N=411
BICR=Blinded Independent Central Review
FDA Clinical/Statistical Review, Figure 1
What are the possible side effects?
The most common side effects of TAGRISSO are diarrhea, skin and nail conditions such as dry skin, rash and infection or redness around the fingernails. TAGRISSO may cause serious side effects, including inflammation of the lungs and injury to the heart. It also may cause harm to a developing fetus.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse events in the pooled clinical trials.
Table 3. Adverse Reactions (Occurring in at Least 10% of Subjects) in the Clinical Trial
| Adverse Reaction | TAGRISSO N=411 (%) |
|---|---|
| Diarrhea | 42 |
| Rash | 41 |
| Dry skin | 31 |
| Nail toxicity | 25 |
| Eye Disorders | 18 |
| Nausea | 17 |
| Decreased appetite | 16 |
| Constipation | 15 |
| Pruritus | 14 |
| Cough | 14 |
| Fatigue | 14 |
| Back pain | 13 |
| Stomatitis | 12 |
| Headache | 10 |
* NCI CTCAE v4.0.
TAGRISSO Prescribing Information, Table 2
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of side effects was similar in men and women.
- Race: The risk of certain side effects, called serious adverse events1, was higher in whites compared to Asians.
- Age: The risk of certain side effects, called Grade 3 and 4 adverse reactions2, were higher in patients 65 years or older compared to those below 65 years.
1Serious adverse event was defined as any event that resulted in one of the following: death, life-threatening event, required hospitalization or extended a current hospital stay, persistent or significant disability/incapacity, or congenital anomaly or birth defect
2According to NCI Common Terminology Criteria for Adverse Events v4.0, Grade 3 refers to severe adverse event and Grade 4 refers to life-threatening or disabling adverse event.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The table below summarizes adverse events for the pooled clinical trials.
Table 4. Toxicity Incidence by Demographics, Pooled Clinical Trials
| All toxicity | Grade 3-4 AEs | Serious AEs | Deaths due to AEs | |
|---|---|---|---|---|
| Sex | ||||
| Male | 96% | 31% | 22.7% | 2.6% |
| Female | 98% | 26.5% | 16.5% | 3.6% |
| Race | ||||
| Asian | 98% | 27.5% | 13.4% | 2% |
| White | 97% | 29% | 27% | 5.3% |
| Age | ||||
| <65 | 97% | 25% | 18.3% | 4% |
| ≥65 | 98% | 31.5% | 18.7% | 2% |
AE=adverse event
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved TAGRISSO based on evidence from two global clinical trials with a total of 411 patients with advanced non-small cell lung cancer with the T790M mutation and whose cancer worsened after treatment with an EGFR-blocking medication. One clinical trial was done in 46 study sites in 10 countries, including Japan, United States, South Korea, Australia, France, Germany, Spain, Italy, Taiwan, and the United Kingdom. The second clinical trial was done in 44 sites in 8 countries, including Canada, Hong Kong, Italy, Japan, South Korea, Spain, Taiwan, and the United States.
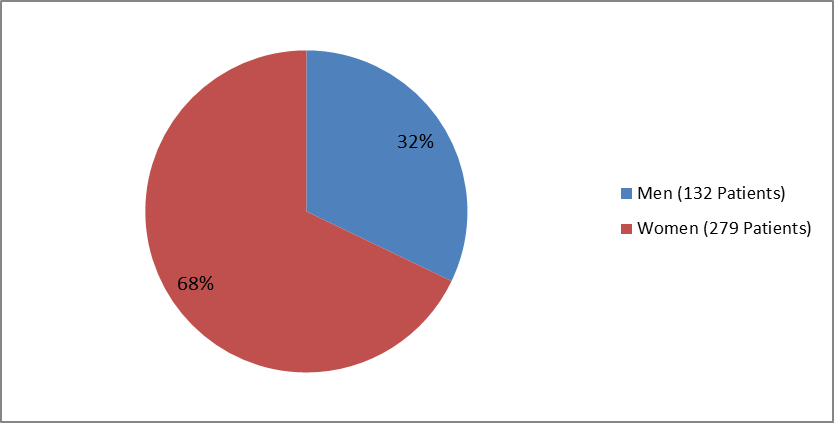
Figure 1 summarizes how many men and women were enrolled in the clinical trials used to evaluate efficacy.
Figure 1. Baseline Demographics by Sex
Clinical Trial Data
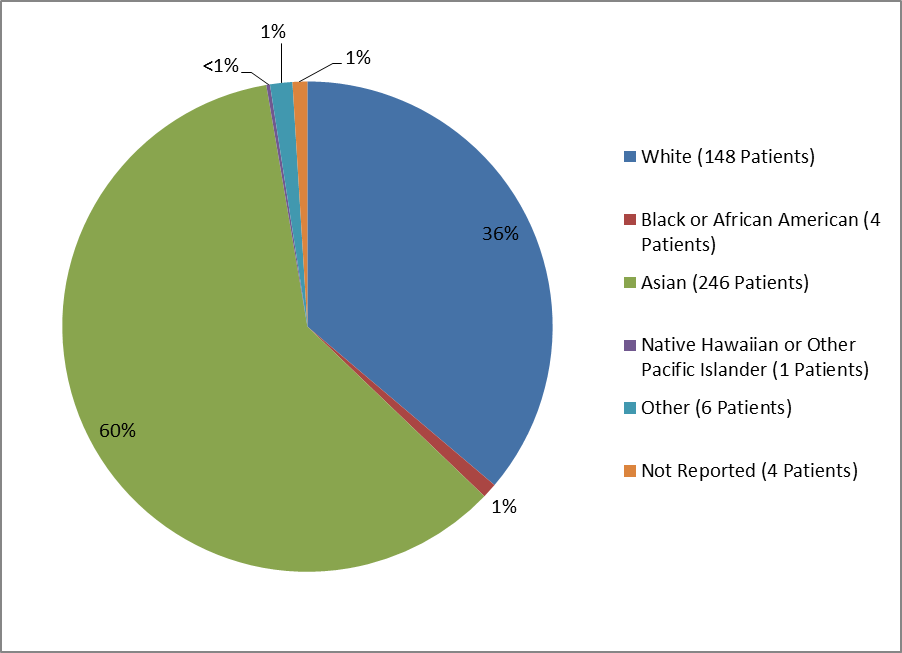
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trials used to evaluate efficacy.
Figure 2. Baseline Demographics by Race
Clinical Trial Data
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 148 | 36% |
| Black or African American | 4 | 1% |
| Asian | 246 | 60% |
| Native Hawaiian or Other Pacific Islander | 1 | <> |
| Other | 6 | 1% |
| Not Reported | 4 | 1% |
Clinical Trial Data
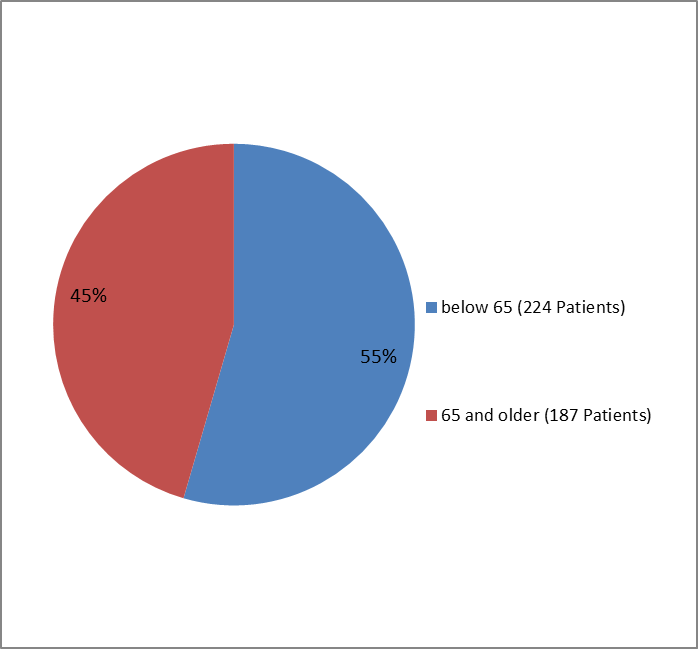
The figure below summarizes how many patients by age group were enrolled in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical Trial Data
Who participated in the trials?
The table below summarizes demographic characteristics of the pooled population.
Table 5. Demographics for Patients in the Pooled Clinical Trials
| Trial 1 N=201 |
Trial 2 N=210 |
Total N=411 |
|
| Sex | |||
| Male | 68 (33.8) | 64 (30.5) | 132 (32.1) |
| Female | 133 (66.2) | 146 (69.5) | 279 (67.9) |
| Race | |||
| White | 76 (38.2) | 72 (34.3) | 148 (36.2) |
| Black or African American | 1 (0.5) | 3 (1.4) | 4 (1) |
| Asian | 114 (57.3) | 132 (62.9) | 246 (60.1) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (0.5) | 1 (0.2) |
| Other | 4 (2) | 2 (1) | 6 (1.5) |
| Not Reported | 4 (2) | 0 | 4 (1) |
| Age | |||
| ˂ 65 | 116 (58) | 108 (51) | 224 (55) |
| ≥65 | 85 (42) | 102 (49) | 187 (45) |
Clinical Trial Data
How were the trials designed?
The benefits and side effects of TAGRISSO were demonstrated in two clinical trials. In both trials, all patients received TAGRISSO.
How were the trials designed?
The efficacy and safety of TAGRISSO were demonstrated in two multicenter, single-arm, open-label clinical trials in patients with metastatic EGFR T790M mutation-positive NSCLC who had progressed on prior systemic therapy, including an EGFR tyrosine kinase inhibitor therapy. All patients were required to have EGFR T790M mutation-positive NSCLC as detected by the cobas® EGFR mutation test and received TAGRISSO 80 mg once daily. The major efficacy outcome measure of both trials was objective response rate (ORR) according to RECIST v1.1 as evaluated by a Blinded Independent Central Review (BICR). Duration of response (DOR) was an additional outcome measure.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION