Drug Trials Snapshot: IBRANCE (palbociclib)
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the IBRANCE Package Insert for complete information.
IBRANCE (palbociclib)
(EYE-brans)
Pfizer, Inc.
Approval date: February 3, 2015
What is the drug for?
IBRANCE is a drug that treats a specific form of advanced breast cancer called ER-positive, HER2-negative (ER+/HER2-) breast cancer in women who have gone through menopause (post-menopausal). It works by blocking the molecules linked to the growth of the cancer cells.
IBRANCE was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
How do I use this drug?
IBRANCE is a capsule that is intended to be taken with a breast cancer treatment called letrozole, another FDA-approved product used to treat certain kinds of breast cancer in post-menopausal women.
What are the benefits of this drug?
The clinical trial that supported the accelerated approval of IBRANCE compared a group of women taking a combination of IBRANCE and letrozole to a group of women taking letrozole alone. Patients taking both IBRANCE and letrozole lived approximately 20.2 months before their tumors worsened, compared to an average of 10.2 months in women who took letrozole alone. Information on overall survival of these women is not available at this time.
What are the benefits of this drug (results of trials used to assess efficacy)?
In the trial, patients in the IBRANCE plus letrozole group lived approximately 20.2 months without their disease pregoressing, compared to approximately 10.2 months in the group receiving letrozole alone.
Table 3 shows the time of Progrssion Free Survival in the IBRANCE and letrozole arm compared to letrozole alone.
Table 3. Efficacy Results of Clinical Trial (Investigator Assessment, Intent-to-Treat Population)
| IBRANCE + letrozole N=84 |
letrozole N=81 |
|
|---|---|---|
| Progression-Free Survival (PFS) | ||
| Number of PFS events (%) | 41 (48.8%) | 59 (72.8%) |
| Hazard Ratio (95% CI) |
0.488 (0.319 to 0.748) |
0.488 (0.319 to 0.748) |
| Median PFS [months] (95% CI) |
20.2 (13.8-27.5) |
10.2 (5.7-12.6) |
CI= Confidence Interval; N= number of patients
Source: FDA Clinical Review, Table 19
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for race and age subgroups.
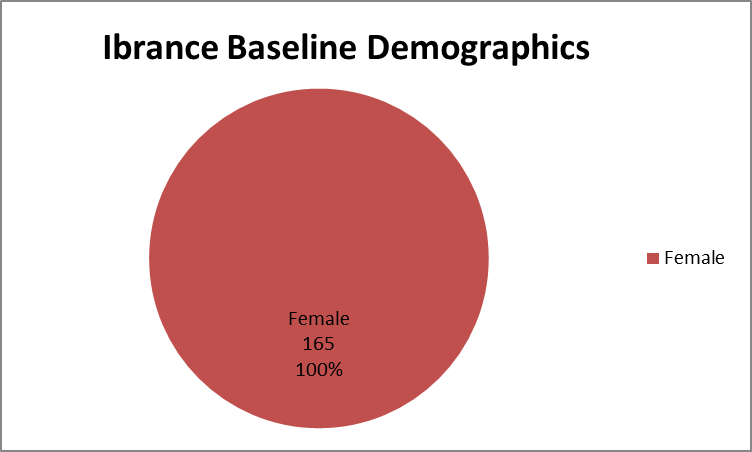
- Sex: The trial included only women.
- Race: Because the number of non-white subjects was limited, it was not possible to determine whether there were any clinically meaningful differences.
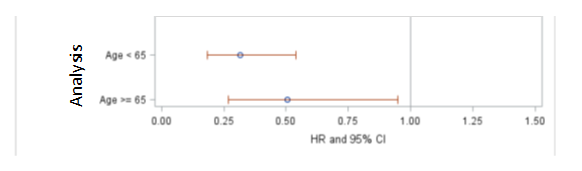
- Age: IBRANCE is similarly effective in patients above and below 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Figure 4 Investigator Assessed Progression-Free Survival Subgroup Analysis
Source: Extracted from FDA Statistical Review, Figure 7
What are the possible side effects?
IBRANCE may cause serious side effects, including low white blood cell counts, infections, and blood clots in the arteries of your lungs (pulmonary embolism).
The most common side effects included low levels of infection-fighting white blood cells called neutrophils (neutropenia), low levels of white blood cells (leukopenia), fatigue, low red blood cell counts (anemia), and upper respiratory tract infections.
What are the possible side effects (results of trials used to assess safety)?
Table 4 below summarizes adverse reactions that occurred in at least 10% of subjects.
Table 4. Adverse Reactions (Occurring in at Least 10% of Subjects) in the Clinical Trial
| Adverse Reaction | IBRANCE + letrozole N=83 (%) |
letrozole N=77 (%) |
|---|---|---|
| Neutropenia | 75 | 5 |
| Leukopenia | 43 | 3 |
| Fatigue | 41 | 23 |
| Anemia | 35 | 7 |
| URI | 31 | 18 |
| Nausea | 25 | 13 |
| Stomatitis | 25 | 7 |
| Alopecia | 22 | 3 |
| Diarrhea | 21 | 10 |
| Thrombocytopenia | 17 | 1 |
| Decreased appetite | 16 | 7 |
| Vomiting | 15 | 4 |
| Peripheral neuropathy | 13 | 5 |
| Asthenia | 13 | 4 |
| Epistaxis | 11 | 1 |
URI=Upper respiratory infection
Source: Extracted from IBRANCE Package Insert, Table 4
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for race and age.
- Sex: The trial included only women.
- ace: Because the number of non-white subjects was limited, it was not possible to determine whether there were any clinically meaningful differences.
- Age: The incidence of overall side effects was similar in patients above and below 65 years of age. Certain side effects—called serious adverse events1 --were seen more frequently in patients age 65 and above compared to those below the age of 65.
1Serious adverse event was defined as any event that resulted in one of the following: death, life-threatening event, required hospitalization or extended a current hospital stay, persistent or significant disability/incapacity, or congenital anomaly or birth defect
Were there any differences in side effects among sex, race and age?
Table 5. Summary of Adverse Events by Age Group
| Category of AE | IBRANCE + letrozole N=83 |
letrozole N=77 |
||
|---|---|---|---|---|
| below 65 years N=46 |
65 years and older N=37 |
below 65 years N=40 |
65 years and older N=37 |
|
| Patients with AEs (%) | 46 (100) | 37 (100) | 33 (83) | 37 (100) |
| SAE (%) | 7 (15) | 11 (30) | 1 (3) | 4 (11) |
| D/C due to AEs (%) | 6 (13) | 6( 16) | 1 (3) | 1 (3) |
| D/C due to TEAEs (%) | 4 (9) | 2 (5) | 1 (3) | 1 (3) |
AE=adverse event, TEAE= Treatment Emergent Adverse Event, D/C=discontinuations
Source: Extracted from Clinical Review, Table 43
WHO WAS IN THE TRIALS?
Who participated in the clinical trials?
The FDA approved IBRANCE based on evidence from a clinical trial of 165 patients with ER+/HER2- advanced breast cancer who had not received any previous treatment for advanced disease. The studies were conducted at 50 sites in 12 countries in North America, Europe, Asia and Africa.
Figure 1 summarizes how many women were enrolled in the clinical trial.
Figure 1. Baseline Demographics by Sex
Source: Extracted from FDA Clinical Review, Table 2
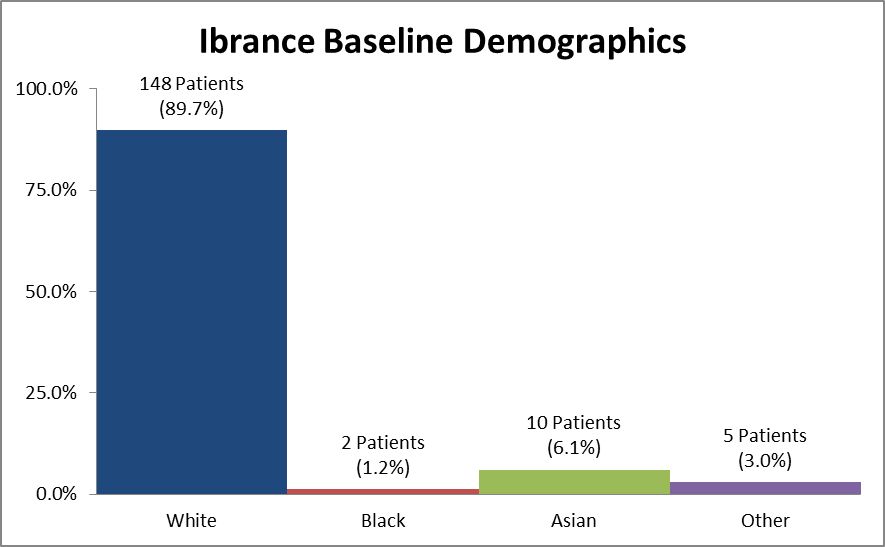
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race
Source: Extracted from FDA Clinical Review, Table 2
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 148 | 89.7% |
| Black | 2 | 1.2% |
| Asian | 10 | 6.1% |
| Other | 5 | 3.0% |
Source: Adapted from FDA Statistical Review, Table 3
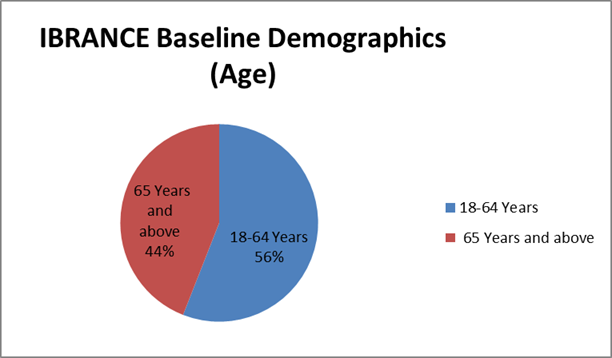
Figure 3 summarizes enrollment by age group in the clinical trial.
Figure 3 Baseline Demographics by Age
Source: Extracted from FDA Clinical Review, Table 14
Who participated in the study?
IBRANCE was evaluated in one clinical trial of women diagnosed with ER-positive, HER2-negative advanced breast cancer who had not received previous treatment for advanced disease. The trial population included 165 women who were randomly assigned to receive IBRANCE in combination with letrozole (84 women) or letrozole alone (81 women).
The trial was conducted at 50 clinical sites in 12 countries, including North America, Europe, Asia, and Africa. The demographic characteristics are summarized in Table 2.
Table 6. Baseline Demographic Characteristics
|
|
IBRANCE plus Letrozole (n=84) |
Letrozole |
|
|---|---|---|---|
|
Age (years), n (%) |
|||
|
18-64 |
47 (56) |
42 (51.9) |
|
|
65 and above |
37 (44) |
39 (48.1) |
|
|
Median (range) |
62.5 (41 to 89) |
64 (38 to 84) |
|
|
Race, n (%) |
|||
|
White |
76 (90.5) |
72 (88.9) |
|
|
Black |
1 (1.2) |
1 (1.2) |
|
|
Asian |
6 (7.1) |
4 (4.9) |
|
|
Other |
1 (1.2) |
4 (4.9) |
|
|
Gender, n (%) |
|||
|
Male |
0 |
||
|
Female |
84 (100) |
81 (100) |
|
|
Country, n (%) |
|||
|
North America |
14 (16.7) |
22 (27.2) |
|
|
Europe/Asia |
69 (82.1) |
59 (72.8) |
|
|
Africa |
1 (1.2) |
0 (0.0) |
|
Source: Extracted from FDA Clinical Review, Table 14
How was the study designed?
The trial compared patients who were randomly assigned to take either a combination of IBRANCE and letrozole or letrozole alone. The treatment continued until the disease progressed, the side effects became too toxic or the patient decided to discontinue the study.
How was the study designed?
The efficacy of IBRANCE was evaluated in one randomized, open-label trial. Randomization was stratified by disease site (visceral versus bone only versus other) and by disease-free survival (more than 12 months from the end of adjuvant treatment to disease recurrence versus 12 months or less from the end of adjuvant treatment to disease recurrence of de novo advanced disease). IBRANCE was given orally at a dose of 125 mg daily for 21 consecutive days followed by 7 days off treatment to comprise a complete cycle of 28 days. Patients received study treatment until progressive disease, unmanageable toxicity, or consent withdrawal. The major efficacy outcome measure of the trial was investigator-assessed Progression-Free Survival (PFS) in Solid Tumors Version 1.0 (RECIST) for both groups.
IBRANCE was evaluated for safety in all the patients who were treated with IBRANCE.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PACKAGE INSERT
MEDICAL REVIEW