Lab Method for Gas Chromatography - Mass Spectrometry (GC-MS) Screening Procedure for the Presence of Diethylene Glycol and Ethylene Glycol in Toothpaste
June 2007
This method for Diethylene Glycol and Ethylene Glycol in toothpaste should be regarded as Level 1 - Urgent Use. The user should verify the performance of the method in their laboratory for each different matrix and pay particular attention to the recommended quality control elements. Users should document with their results the version or date of the method used.
PURPOSE:
This procedure was developed at the Forensic Chemistry Center (FCC) to examine toothpaste for the presence of Diethylene Glycol (and a related substance of similar toxicity, Ethylene Glycol) at levels of 1 mg/g (0.1% by weight) and above.
SCOPE:
This procedure has been shown to be capable of detecting both analytes at a level of 0.5 mg/g a variety of different brands of toothpaste.
RESPONSIBILITY:
It is the responsibility of the analyst to note any modifications to or deviations from this procedure in the documentation associated with the analysis.
DEFINITIONS AND ACRONYMS:
SAFETY CONSIDERATIONS:
Appropriate safety measures should be employed when working with chemicals, pressurized gases and the instrumentation which is employed. Generally, these will be defined in each laboratory's safety plan. No extraordinary precautions are necessary here.
EQUIPMENT AND SUPPLIES:
- Agilent 5975i GC-MS system equipped with a 30 m Stabilwax (crossbond Carbowax) capillary column (or equivalent) or other mass spectrometer system.
- Laboratory centrifuge capable of applying 5000 g to 15 mL centrifuge tubes.
- 15-mL polypropylene centrifuge tubes with screw caps.
REAGENTS AND STANDARDS:
- Extraction Solvent: Water followed by the addition of Acetonitrile
- 1,3-Propanediol
- For use as an internal standard. Prepare a stock solution at 5.0 mg/mL in 50% aqueous acetonitrile.
- Add 0.050 mL of this solution to 0.500 mL of each sample extract in the autosampler vial prior to analysis.
- Diethylene Glycol
- Prepare a stock solution of 10.0 mg/mL in 50% aqueous acetonitrile.
- Ethylene Glycol.
- Prepare a stock solution of 10.0 mg/mL in 50% aqueous acetonitrile.
- Mixed Standard Spiking Solution
- Mix together equal volumes of the stock standards, (3) and (4) above.
QC ELEMENTS:
A Method Blank which consists of 10 mL of extraction solvent taken through the entire procedure including the addition of the internal standard should be evaluated to make sure that there is no contamination from the reagents or the containers.
Condition the System at the beginning of each set of samples by making two injections of the high standard (see below).
A Low Standard, which consists of each analyte at 0.10 mg/mL, should be analyzed at the beginning and the end of the sample set to show that the necessary sensitivity was attained by the instrument.
A High Standard at 0.50 mg/mL of each analyte is analyzed at the beginning and the end of the sample set to provide a basis for semi-quantitative evaluation and to monitor the amount of drift during the analysis of the set of samples.
A Fortified Control Sample consists of a representative of the type of samples which are being analyzed and which has been fortified with each analyte (viz. Diethylene Glycol and Ethylene Glycol) at a level of 1 mg/g (or 0.1 % by weight). Analysis of this fortified control sample must show that the compounds are present. This serves to demonstrate effective system performance at the target level. See Sample Fortification, below.
PROCEDURE:
-
Sample Preparation
Weigh approximately 1.0 g of toothpaste into a 15-mL polypropylene centrifuge tube.
Add 5 mL of water.
Mix well to thoroughly disperse the entire sample. Use of a vortex mixer (e.g. Genie 2, Fisher Scientific) can assist this process. Foam will probably be formed.
Then add 5 mL of acetonitrile in two portions with thorough mixing between each addition. The acetonitrile will suppress the foam.
Centrifuge for 10 minutes at 5000 g or greater.
Transfer 0.50 mL of the supernatant to an autosampler vial. Add 0.050 mL of the internal standard (i.e. 1,3-propanediol at 5.0 mg/mL).
Analyze.
-
Instrument Parameters
This procedure suggests that the GC be operated in the split mode (20:1) to improve peak shape and reduce the burden of sample components on the chromatographic system.
GC Conditions Column: Restek Stabilwax (30 m × 0.25 mm id × 0.25 µm df) with a 5 m retention gap (or equivalent) Inlet Temperature: 250 °C Transfer Line Temperature: 250 °C Injection Mode: Split (20:1) Injection Volume: 1 µL Carrier Gas Flow: He at 35 cm/sec (constant flow) Oven Program: 100 °C (1 min) to 250 °C at 10 °C/min (Hold 4.00 min) for a total run time of 20 min. Use 50% aqueous acetonitrile in the wash vials for cleaning the syringe between injections. This removes sample residue that may not be soluble in other wash solvents. This will reduce injector errors.
Note: Alterations to the GC temperature program may be used to help overcome interferences. Significant additional resolution may be obtained by decreasing the temperature ramp over relevant portions of the chromatogram. This will alter retention times for the analytes and they would need to be re-established.MS Conditions (Full Scan Mode) Tune: Standard Spectrum autotune Scan Range: 29 - 400 amu Sampling Rate: 2 (Scan Rate of about 4 scans/sec) Threshold: 100 Solvent Delay: 4 minutes MS Temp: 230 °C (Source); 150 °C (Quad) MS Conditions (SIM Mode)
This is provided primarily for information only since it is unlikely that SIM will need to be utilized.
There are a lot of potential interferences with the low mass ions. The higher mass ions are more useful even though, generally, the sensitivity is relatively poor. No significant molecular ion is observed for 1,2-propanediol, 1,3-propanediol, diethylene glycol or glycerin.
Group Start Time a
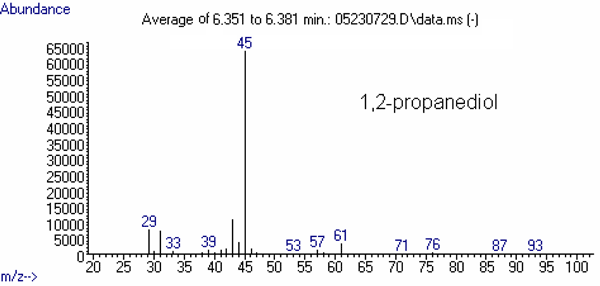
(min)Selected Ions b 1 2 3 4 1,2-Propanediol 4.0 31
(11) c43
(15)45
(100)61
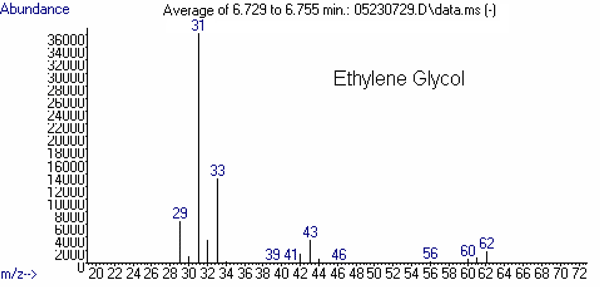
(5)Ethylene Glycol 6.6 31
(100)33
(35)43
(8)62 (M) d
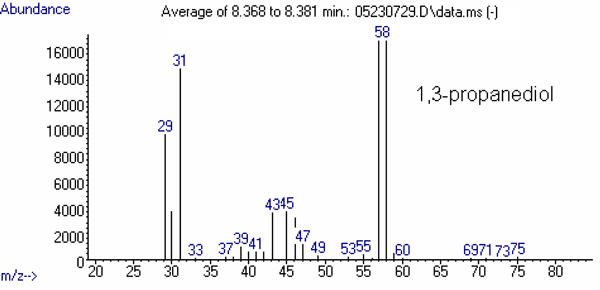
(4)1,3-Propanediol 7.5 31
(100)43
(22)57
(73)58
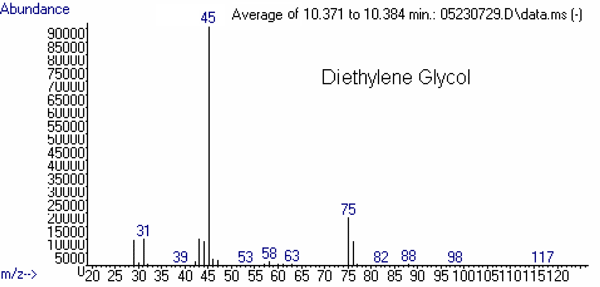
(68)Diethylene Glycol 9.0 31
(11)45
(100)75
(24)76
(12)Glycerin 12.0 31
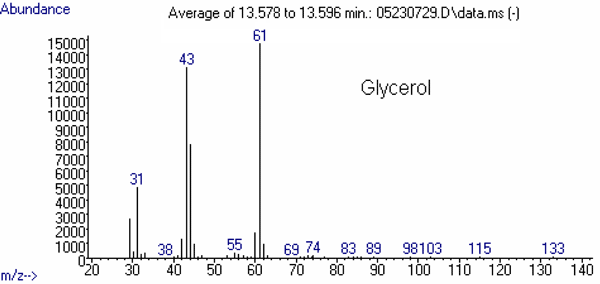
(40)43
(78)60
(13)61
(100)a Start Times may need to be adjusted based upon the retention time of standards on your system.
b Dwell times should be adjusted to produce a cycle time of about 4 scans/sec
c Percent relative abundance with respect to the ion of highest abundance.
d M is the Molecular Ion. -
Peak Identification
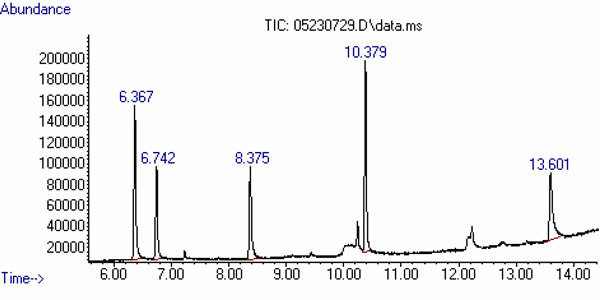
The approximate retention times of the analytes are:
Compound Retention Time
(min)Comment 1,2-Propanediol 6.4 This is a related compound. Ethylene Glycol 6.8 1,3-Propanediol 8.4 Internal Standard Diethylene Glycol 10.4 Glycerin 13.7 This is a common component of toothpaste. These need to be confirmed on your system.
Figure 1 is a standard chromatogram showing the analytes mentioned above.
The mass spectrum of each of these compounds is presented in Figures 2 through 4.
In full scan mode, the criteria for identification of target analytes include the agreement of the retention times with those of the standards to within 0.05 minute. Also, the mass spectra need to correspond to those of the standards with no significant peaks absent. There may be additional peaks present due to overlap with other components but this should be examined carefully.
In SIM mode, the criteria for identification of target analytes include the agreement of the retention times with those of standards to within 0.05 minute. Mass spectral confirmation of an analyte is based upon the ratios of the integrated areas for selected ions to the integrated area of the most abundant of the selected ions being tracked. The criterion is that each ratio (as a percentage) corresponds to that observed for a standard to within 10 units.
Because there are few unique ions among these analytes and, as a consequence, there exists the prospect for significant interferences, confirmation of identity may be problematic. Reference is made below (See G. References) to a description of an approach to confirmation which consists of preparing silyl derivatives of the analytes for analysis by GC-MS under different conditions.
-
Sample Fortification and Mixed Standard Preparation
A Mixed Standard Spiking solution is prepared by mixing equal portions of the two stock standard solutions to create a solution which is 5.0 mg/mL in each analyte.
Spike Preparation
Weigh a 1.0 g portion of toothpaste into a 15-mL centrifuge tube. A previously analyzed, representative example of the product which was found to be uncontaminated with the targets may be used.
Alternatively, select one of the samples which is about to be analyzed and spike a portion of it. This would have to be repeated with a different sample if it turns out that analysis of this sample without fortification gave evidence of contamination.
Add 200 µL of the Mixed Standard Spiking solution directly to the representative uncontaminated sample and proceed with the method. The spike must be observed to provide a basis for declaring that samples which provide signals below a certain threshold contain less than 1.0 mg/g of either target.
High Standard
Dilute the Mixed Standard Spiking Solution to 0.5 mg/mL with 50% aqueous acetonitrile. Place 500 µL of this solution in a vial and add 50 µL of the Internal Standard solution.
Low Standard
Dilute the Mixed Standard Spiking Solution to 0.10 mg/mL. Place 500 µL of this solution in a vial and add 50 µL of the Internal Standard solution.
-
Reporting
In the event that the analytes were observed in the representative control sample that was fortified at 1.0 mg/g and no analyte signals were observed in the samples at the levels which were observed in the fortified control, then the samples are not contaminated with ethylene glycol or diethylene glycol at levels in excess of 0.1 % by weight (i.e. 1 mg/g).
If it is clear that one or more of the analytes are present in the samples (based on the identification criteria above) and at levels greater than or equal to 1.0 mg/g, then the result should be reported as positive.
-
Obtaining a Semi-quantitative Estimate of Target Analytes
Choose an ion to use for estimating the amount of the target analyte (for example, m/z = 75 for diethylene glycol and m/z = 62 for ethylene glycol). Using the High Standard (0.50 mg/mL) which was run at the beginning of the set of samples and the High Standard that was run at the end of the set of samples, calculate the average peak area for the selected ion.
Apply the following formula:
Concentration in Sample Extract (µg/mL)
= [Area (Test Sample) / Average Area (High Standard)] × 0.50mg/mL.Then,
Concentration in Sample (µg/g)
= Concentration in Test Sample Extract (mg/mL) × 10.0 mL × [1 / Sample Wt. (g)]And, finally,
Concentration in Sample (% by weight) = Concentration in Sample (mg/g) × 1/10
If the internal standard areas differ by more than 20% between the test sample and the standard then adjust the area of each analyte response by dividing by the integrated area of the internal standard (using the ion at 58 amu). Use these ratios in place of the integrated areas above. Consider re-analysis if the difference is extremely large since that may indicate a partially clogged syringe or other instrumental problem.
Finally, if the signal from the sample ions is more than 10 × larger than the signal from the High Standard, then do additional dilution of the extract to bring the concentration of the analytes into the range of the High Standard.
As a general rule-of-thumb, the closer the analyte signal from the unknown is to the analyte signal from the standard, the better the estimate of the unknown concentration. So, if better numbers are required, include additional standards and re-analyze the extracts in question.
-
References
K. J. Mulligan: No. 4042 - A Procedure to Determine Diethylene Glycol (2,2'-oxybisethanol) and Ethylene Glycol (1,2-ethanediol) in Glycerin and Selected Products, Laboratory Information Bulletin 12(7), DHHS (1996).
AUTHORS
Jonathan Litzau, Forensic Chemistry Center (FCC)
Kevin Mulligan, Forensic Chemistry Center (FCC)
Figure 1. Total-ion Chromatogram (TIC) of the Analytes and
Related Compounds at a Concentration of about 100 mg/mL of each.
| Compound | Retention Time (min) |
Comment |
|---|---|---|
| 1,2-Propanediol | 6.37 | Related Material |
| Ethylene Glycol | 6.74 | Target |
| 1,3-Propanediol | 8.38 | Internal Standard |
| Diethylene Glycol | 10.38 | Target |
| Glycerin | 13.60 | Probable Component of Toothpaste |
Figure 2: Mass Spectra of the Targets: Ethylene Glycol and Diethylene Glycol.
Figure 3: Mass Spectrum of the Internal Standard
Figure 4: Mass Spectra of Other Relevant Substances