Drug Trials Snapshots: Zinbryta
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ZINBRYTA Prescribing Information for complete information.
ZINBRYTA (daclizumab)
(zin-bry-tuh)
Biogen Inc
Approval date: May 27, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ZINBRYTA is a drug for the treatment of relapsing forms of multiple sclerosis (MS) in patients who have not responded adequately to at least 2 other treatments for MS.
How is this drug used?
ZINBRYTA is given once every month by injection under the skin (subcutaneous injection) of the thigh, belly, or back of the upper arm.
What are the benefits of this drug?
Patients treated with ZINBRYTA had fewer worsening episodes (relapses) of MS in one year.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the efficacy results for Trial 1.
Table 2. Clinical and MRI Results of Trial 1
ZINBRYTA | AVONEX 3 0 mcg IM Once Weekly N=922 | p-value | |
|---|---|---|---|
| Clinical Results1 | |||
| Annualized relapse rate Relative reduction Proportion Relapse Free | 0.216 45% 67% | 0.393 | |
| Proportion with 12-week confirmed disability progression | 16% | 20% | 0.16 |
| MRI Results 2 | |||
| Mean number of new or newly enlarging T2 hyperintense lesions Relative reduction | 4.31 54% | 9.44 | |
1Values refer to results up to 144 weeks
2MRI analysis used evaluable dataset and values reflect results at 96 weeks
ZINBRYTA Prescribing Information
Table 3. Clinical and MRI Results of Trial 2
ZINBRYTA | Placebo N=204 | p-value | |
|---|---|---|---|
| Clinical Results1 | |||
| Annualized relapse rate Relative reduction Proportion Relapse Free | 0.211 54% 81% | 0.458 64% | |
| Proportion with 12-week confirmed disability progression3 Relative risk reduction | 6% 57% | 13% | |
| MRI Results2 | |||
| Mean number of new or newly enlarging T2 hyperintense lesions1 Relative reduction |
| 8.1 | |
| Mean number of new T1 Gd-enhancing lesions Relative reduction | 1.46 69% | 4.79 | |
1 Values refer to results at 52 weeks
2MRI analyses used evaluable dataset for each endpoint; T1 Gd-enhancing: MRI intensive population, between 8-24 weeks
3The proportion of patients with 12-week confirmed disability progression was an exploratory measure in Study 2. As the proportion of patients with 12-week confirmed disability was used as a key secondary outcome in Study 1, and is one of the main outcome measures in MS studies, the disability progression results are presented for Study 2. The nominal p value for that comparison, p=0.02, is not adjusted for multiple comparisons
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ZINBRYTA worked similarly in men and women
- Race: The majority of patients in the trials were white. Differences in response to ZINBRYTA among races could not be determined.
- Age: All patients in the trials were below age 65. Differences in response to ZINBRYTA between patients above and below age 65 could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
Subgroup differences in relapse rates in Trials 1 and 2 are presented in the tables below.
Table 3. Analysis of the Primary Endpoint by Demographic Subgroups (Trial 1)
| Avonex | ZINBRYTA | ||
|---|---|---|---|
| N=919 | N= 922 | ||
| Sex, Men | |||
| n | 295 | 294 | |
| Adjusted annualized relapse rate | 0.401 | 0.186 | |
| Rate ratio (95% CI) | 0.46 (0.35, 0.62) | ||
| Sex, Women | |||
| n | 627 | 625 | |
| Adjusted annualized relapse rate | 0.386 | 0.227 | |
| Rate ratio (95% CI) | 0.59 (0.49, 0.71) | ||
| Age, ≤ 35 | |||
| n | 449 | 451 | |
| Adjusted annualized relapse rate | 0.505 | 0.205 | |
| Rate ratio (95% CI) | 0.41 (0.32, 0.51) | ||
| Age, > 35 | |||
| n | 473 | 468 | |
| Adjusted annualized relapse rate | 0.315 | 0.233 | |
| Rate ratio (95% CI) | 0.74 (0.59, 0.92) | ||
| Race-White | |||
| n | 828 | 823 | |
| Adjusted annualized relapse rate | 0.394 | 0.213 | |
| Rate ratio (95% CI) | 0.54 (0.46, 0.64) | ||
| Race- non-White | |||
| n | 94 | 96 | |
| Adjusted annualized relapse rate | 0.377 | 0.241 | |
| Rate ratio (95% CI) | 0.64 (0.38, 1.08) | ||
FDA Statistical review
Table 4. Analysis of the Primary Endpoint by Demographic Subgroups (Trial 2)
| Placebo | ZINBRYTA | |
|---|---|---|
| N=196 | N=201 | |
| Sex, Men | ||
| n | 73 | 65 |
| Adjusted annualized relapse rate | 0.43 | 0.13 |
| Rate ratio (95% CI) | 0.22 (0.10, 0.48) | |
| Sex, Women | ||
| n | 123 | 136 |
| Adjusted annualized relapse rate | 0.32 | 0.22 |
| Rate ratio (95% CI) | 0.66 (0.40, 1.07) | |
| Age => | ||
| n | 90 | 99 |
| Adjusted annualized relapse rate | 0.46 | 0.18 |
| Rate ratio (95% CI) | 0.32 (0.18, 0.56) | |
| Age > 35 | ||
| n | 106 | 102 |
| Adjusted annualized relapse rate | 0.28 | 0.20 |
| Rate ratio (95% CI) | 0.68 (0.38, 1.20) | |
| Race-White | ||
| n | 189 | 195 |
| Adjusted annualized relapse rate | 0.430 | 0.208 |
| Rate ratio (95% CI) | 0.48 (0.33, 0.71) | |
| Race-non-White | ||
| n | 7 | 6 |
| Adjusted annualized relapse rate | 1.175 | 0.364 |
| Rate ratio (95% CI) | 0.31 (0.06, 1.67) | |
FDA Statistical review
What are the possible side effects?
ZINBRYTA may cause serious and life threatening liver injury, immune-mediated disorders including allergic reactions, infections, and depression and suicide.
The most common side effects of ZINBRYTA are nasopharyngitis, upper respiratory tract infection and rash.
ZINBRYTA may cause serious adverse reactions including
- severe liver injury, including life-threatening and fatal events, liver failure, and autoimmune hepatitis
- Immune mediated disorders including skin reactions, lymphadenopathy, non-infectious colitis , sarcoidosis
- Hypersensitivity reactions (anaphylaxis and angioedema) increased risk of infection and depression and/or suicidal ideation
The tables below summarize the adverse reactions that occurred at least 2% more frequently in patients treated with ZINBRYTA in Trials 1 and 2.
Table 5. Adverse Reactions in Adults With RMS That Occurred at least 2% More Frequently for ZINBRYTA Than AVONEX (Trial 1)
| Adverse Reaction | ZINBRYTA 150 mg SQ Every 4 Weeks N=919 % | AVONEX 30 mcg IM Once Weekly N=922 % |
| Nasopharyngitis | 25 | 21 |
| Upper respiratory tract infection1 | 17 | 14 |
| Rash2 | 11 | 4 |
| Influenza | 9 | 6 |
| Dermatitis3 | 9 | 2 |
| Oropharyngeal pain | 8 | 4 |
| Bronchitis | 7 | 5 |
| Eczema4 | 5 | 2 |
| Lymphadenopathy | 5 | |
| Tonsillitis | 4 | 2 |
| Acne | 3 |
1 includes upper respiratory tract infection and viral upper respiratory tract infection
2 includes erythematous rash, exfoliative rash, macular rash, maculopapular rash, papular rash, pruritic rash, rash, and vesicular rash
3 includes allergic dermatitis, atopic dermatitis, bullous dermatitis, dermatitis, exfoliative dermatitis, and seborrheic dermatitis
4 includes dyshidrotic eczema, eczema, and nummular eczema
ZINBRYTA Prescribing Information
Table 6. Adverse Reactions in Adults with RMS That Occurred at least 2% More Frequently for ZINBRYTA than Placebo (Trial 2)
| Adverse Reaction | ZINBRYTA 150 mg SQ Every 4 Weeks N=208 % | Placebo N=204 % |
| Upper Respiratory Tract Infection | 9 | 7 |
| Depression1 | 7 | 2 |
| Rash2 | 7 | 3 |
| Pharyngitis | 6 | 4 |
| Increased ALT | 5 | 2 |
| Rhinitis | 4 | 1 |
| Anemia | 3 | |
| Pyrexia | 3 | |
| Increased AST | 3 | |
| Dermatitis3 | 3 |
1 includes depressed mood and depression
2 includes erythematous rash, exfoliative rash, macular rash, maculopapular rash, papular rash, pruritic rash, rash, and vesicular rash
3 includes allergic dermatitis, atopic dermatitis, bullous dermatitis, dermatitis, exfoliative dermatitis, and seborrheic dermatitis
ZINBRYTA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The rate of side effects was similar in men and women.
- Race: The majority of patients in the trials were white. Differences in side effects among races could not be determined.
- Age: All of the patients in the trials were below age 65. Differences in side effects between patients above and below age 65 could not be determined.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The table below summarizes the most common adverse reaction that occurred in both trials (upper respiratory tract infection) by subgroup
Table 7. Upper Respiratory Tract Infection by Subgroups in ZINBRYTA treated Participants
| Demographic Parameters | ZINBRYTA (N=1127) | Avonex (N=922) | Placebo (N=204) |
|---|---|---|---|
| Patients with AR* n/N (%) | Patients with AR* n/N (%) | Patients with AR* n/N (%) | |
| Sex | |||
| Men | 45/362 (12) | 40/295(14) | 1/76 (1) |
| Women | 122/765 (16) | 84/627(13) | 14/128 (11) |
| Age Group | |||
| 40> | 104/704 (15) | 78/579 (13) | 9/126 (7) |
| 40 years and older | 63/423 (15) | 46/343(13) | 6/78 (7) |
| Race | |||
| White | 157/1025 (15) | 118/828 (14) | 15/197(3) |
| All Other | 10/102 (10) | 6/94(6) | 0/7(0) |
* Adverse Reaction: Upper Respiratory Tract Infection
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA based the approval of ZINBRYTA on evidence from 2 clinical trials with 2253 patients with relapsing multiple sclerosis. The patients lived in the United States, Canada, Europe, Asia and South America.
Figure 1 summarizes how many men and women volunteered to be in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical trial data
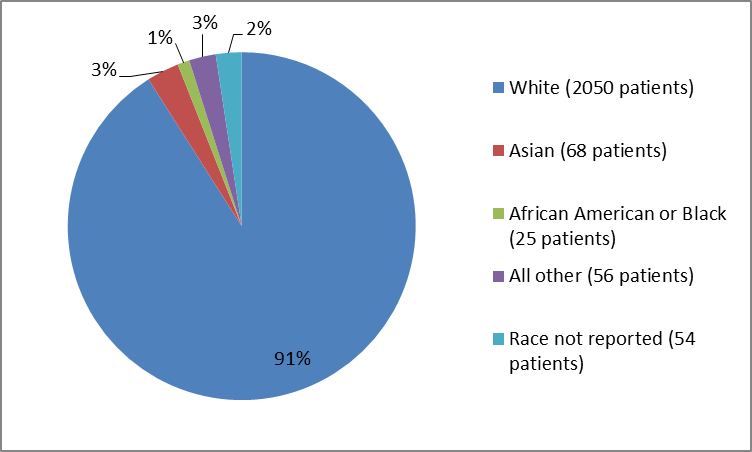
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical trial data
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 2050 | 91% |
| Black or African American | 25 | 1% |
| Asian | 68 | 3% |
| All Other | 56 | 3% |
| Not reported | 54 | 2% |
Clinical trial data
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in clinical Trials 1 and 2 combined.
Table 8. Baseline Demographics
| Demographic Parameters | ZINBRYTA (N=1127) | Avonex (N=922) | Placebo (N=204) | Total (N=2253) |
|---|---|---|---|---|
| Sex | ||||
| Men | 362 (32%) | 295 (32%) | 76 (37%) | 733 (33%) |
| Women | 765 (68%) | 627 (68%) | 128 (63%) | 1520 (67%) |
| Age | ||||
| Median (years) | 36 | 36 | 37 | 36 |
| Min, Max (years) | 18, 56 | 18, 56 | 19, 55 | 18, 56 |
| Age Group | ||||
| 18-39 years | 704 (62%) | 579 (63%) | 126 (62%) | 1409 (63%) |
| 40-56 years | 423(38%) | 343 (37%) | 78 (38%) | 844 (37%) |
| Race | ||||
| White | 1025 (91%) | 828 (90%) | 197 (97%) | 2050 (91%) |
| African American or Black | 13 (1%) | 12 (1%) | 0 | 25(1%) |
| Asian | 33 (3%) | 28 (3%) | 7 (3%) | 68 (3%) |
| American Indian or Alaska Native | 0 | 1 ( | 0 | 1 ( |
| Other | 27(3%) | 28 (3%) | 0 | 55 (2%) |
| Race not reported | 29 (3) | 25 (3) | 0 | 54 (2%) |
| Region | ||||
| United States and Canada | 118(10%) | 118 (13%) | 0 | 236 (10%) |
| Other* | 1109 (90%) | 604 (87%) | 204 (100%) | 1917 (90%) |
*From countries in Europe, Asia and South America.
Clinical trial data
How were the trials designed?
There were two trials that evaluated the benefits and side effects of ZINBRYTA.
In Trial 1, which was 96 weeks in duration, patients had injections with ZINBRYTA injections every four weeks or with an approved drug for MS called Avonex every week. In Trial 2, which was 52 weeks in duration, patients had injections with ZINBRYTA or a similar inactive drug every four weeks.
Neither the patients nor the health care providers knew for sure which treatment was being given until after the trials were completed. The trials counted the number of episodes when the patients' symptoms became worse ( MS relapses) each year and compared the number for ZINBRYTA to the numbers for Avonex and placebo.
How were the trials designed?
ZINBRYTA trials include 2 double-blind, randomized trials.
In Trial 1, ZINBRYTA given SC every 4 weeks was compared to AVONEX 30 mcg IM weekly. The treatment lasted 96 weeks and the primary endpoint was the annualized relapse rate (ARR).
In Trial 2, ZINBRYTA given SC every 4 weeks was compared to placebo. The treatment lasted 52 weeks and the primary endpoint was the annualized relapse rate (ARR) at week 52.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.